Pityriasis rosea is an acute self-limiting papulo-squamous skin disorder of unknown aetiology. Its occurrence is ubiquitous. In different studies the incidence of pityriasis rosea was recorded to be between 0.39-4.80 per 100 dermatology patients [1,2]. Though self-limiting by 3-6 weeks, the clinical course in some patients may be prolonged over several months and recurrence is known to occur. The presence of generalized skin lesions results in considerable anxiety among patients with pityriasis rosea and there is a significant psychological impact upon parents of affected young children [3]. Post-inflammatory hyperpigmentation may also result in significant cosmetic concern among young patients. Recent data suggest the possibility that pityriasis rosea may pose a risk for spontaneous abortion in pregnant women [4]. Thus an effective treatment for this condition is essential.
The exact aetiology of pityriasis rosea is not known. Seasonal occurrence, clustering of cases and presence of occasional prodromal symptoms suggests the possibility of an infectious agent involved in its pathogenesis [5–7]. Use of new garments or old garments in storage for prolonged period have been suggested as precipitating factors for pityriasis rosea, indicative of a transmissible infectious agent [8]. A chance observation of improvement of skin lesions of pityriasis rosea in two patients who were given erythromycin for upper respiratory tract infections, also confirms this hypothesis [9]. It has already been established that drugs like allopurinol, arsenic, bismuth, barbiturate, gold, hydrochlorothiazide, organic mercurials, nimesulide, d-penicillamine, clonidine, isotretinoin and ketotifen can cause eruptions resembling pityriasis rosea [10–12]. Ampicillin and systemic corticosteroids have been found to exacerbate PR [11]. Isolated cases of pityriasis rosea like rashes have also been reported following administration of captopril, metronidazole and omeprazole [13–15].
Recently DNA of human herpes virus 6 and 7 (HHV-6 and HHV-7) have been isolated from lesional and non-lesional skin, peripheral blood mononuclear cells, serum and saliva samples of patients with pityriasis rosea [16]. In view of probable involvement of HHV-6 in its pathogenesis, anti-viral drugs may be useful in the treatment of pityriasis rosea. Few trials of acyclovir in pityriasis rosea has shown faster clearance of skin lesions and shortened duration of the disease [17–19] Based on the hypothesis of infectious aetiology in pityriasis rosea, as well as several circumstantial evidences for this, a trial on efficacy of acyclovir in this disorder was conducted.
To determine the efficacy of acyclovir, an anti-viral drug, in the treatment of pityriasis rosea.
Materials and Methods
A randomized, double blind, placebo controlled trial was conducted to determine the efficacy of acyclovir, an anti-viral drug, in the treatment of pityriasis rosea. Seventy-three patients suffering from pityriasis rosea were recruited from the out-patients’ section of the department of dermatology of a tertiary health care hospital in South India during the period of November 2006 to May 2008.
Inclusion criteria: Clinically diagnosed cases of pityriasis rosea, irrespective of age and sex were included in the study.
Exclusion criteria: Patients who had taken some form of systemic therapy for pityriasis rosea (e.g. corticosteroids, erythromycin) before attending the hospital and those with major systemic illnesses including renal impairment were excluded.
Informed consents were taken from all the patients and the parents of children. Clearance from institutional ethical committee, in accordance with the revised Helsinki Declarartion was obtained.
Procedure: Detailed history of the illness regarding onset, evolution, duration, symptoms, systemic features, recurrence, history of contact and associated factors like socioeconomic status, history of drug intake, use of new clothing, along with other epidemiological data were recorded in the scheduled proforma for comparison with available published data. Complete hemogram and urine analysis were done for all patients. Skin biopsy for histopathological examination from the herald patch or secondary rash was performed with patient’s consent. Venereal disease research laboratory (VDRL) test was done, whenever necessary, in young sexually active adult patients.
Therapy of the patients was advised by a dermatologist other than the chief investigator. Thirty-eight randomly selected (simple randomization by lottery) patients were started on oral acyclovir (800 mg 5 times/day for adults and 20 mg /kg / dose, 4 times / day for children for duration of 7 days). Acyclovir was administered irrespective of the duration of the disease. This was based on the report that HHV-6 DNA had been detected in peripheral blood mononuclear cells during acute illness and also up to 2 months in 78% cases [12]. Thirty-five patients were prescribed placebo (tablet vitamin C 100 mg, 5 times/day for adults and 50 mg 4 times / day for children for a duration of 7 days). Vitamin C was used as the placebo in this trial. It is essential for formation of hydroxyproline, an integral constituent of collagen. So, it is needed for growth of collagen fibers in the dermis, subcutaneous tissue, cartilage, bone, teeth and blood vessel walls [20]. In pityriasis rosea, most of the histopathological changes are seen in the epidermis. Therefore administration of vitamin-C as placebo is unlikely to influence the rate of resolution of skin lesions of pityriasis rosea. The patients were kept unaware of the therapeutic group to which they belong to (acyclovir or placebo). The chief investigator and the dermatologist assigned to give treatment were also unaware of the drug prescribed to individual patients. Symptomatic treatment and antihistamines were administered whenever necessary. Patients in both the groups were evaluated clinically after 7 and 14 days.
Skin lesions were evaluated as follows [17]:
Regressed; if erythema had decreased or disappeared in all lesions leaving desquamation or pigmentation.
Partially regressed; if erythema was decreased in ≥ 50% of the lesions.
Unchanged; if decrease in erythema was recorded in < 50% of the lesions.
New lesions appearing during treatment, if any, were recorded at 7th and 14th days. The type and severity of any systemic symptoms was evaluated during these visits. The time taken for clearance of lesions was recorded. Therapeutic effect on the course of the disease was assessed. Effect of early treatment upon the total duration of the disease was evaluated.
Statistical Analysis
The collected data was analyzed by using standard statistical methods. Data on patients in the acyclovir and placebo group were compared using Student t-test. Efficacy of treatment in the acyclovir group and the placebo group was compared using Z-test. Significance of the pretreatment duration of illness on disease outcome was measured by t-test. Mean and standard deviation (± SD) was calculated in each group and the level of significance was determined.
Results
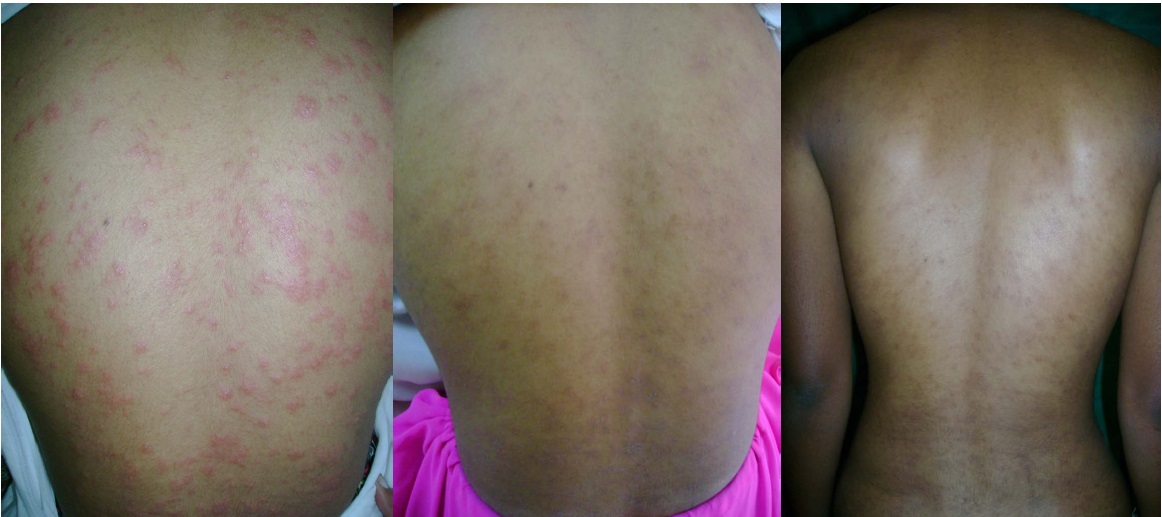
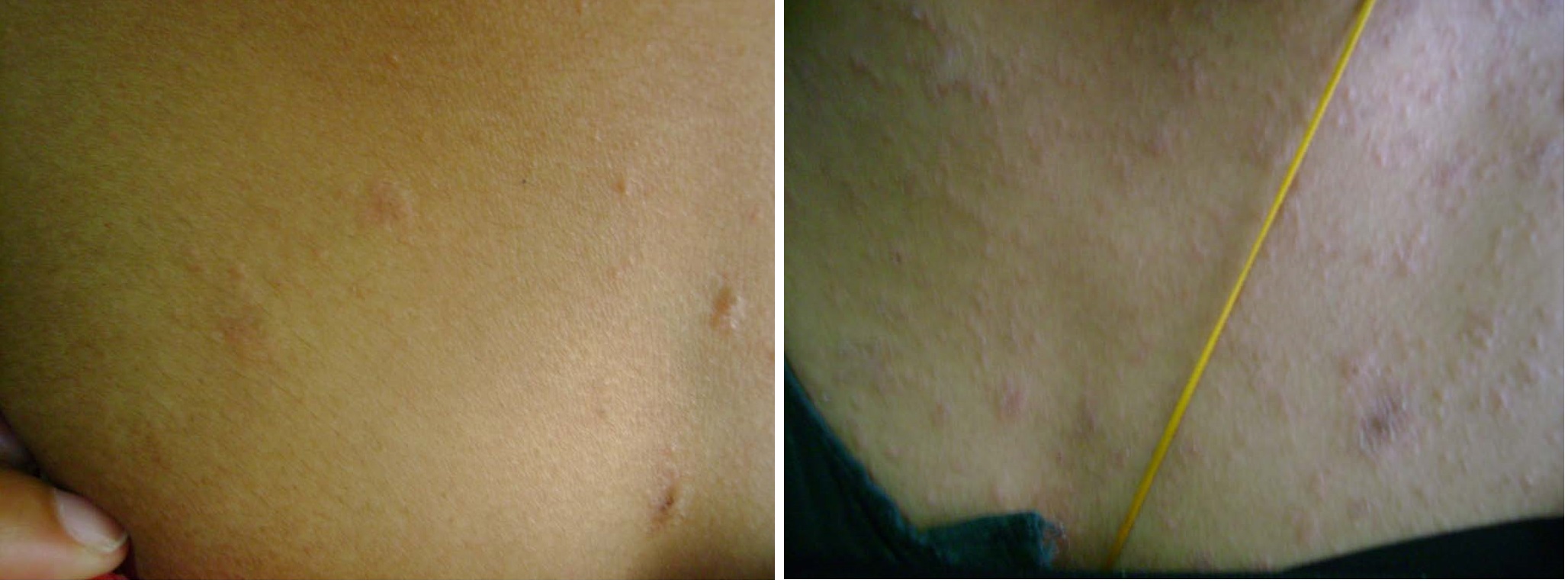
Follow up data of 60 patients (30 in each group) were available and these were included in the statistical analysis. There was no significant age and gender difference among acyclovir and the placebo group (p>0.05). Therapeutic response of patients in the acyclovir [Table/Fig-1a-c] and the placebo groups [Table/Fig-2a&b] have been presented in [Table/Fig-3]. The difference between two groups was statistically significant both on the 7th and 14th day after the first visit. The data presented in [Table/Fig-4] shows that patients in the placebo group developed new lesions more than the acyclovir group on both follow ups. Patients in the acyclovir group were divided in to two sub-groups based on whether the patient received treatment within the first week of onset of lesions, or beyond this period. There was no statistically significant difference (p=0.2874) between the two subgroups regarding the time taken for clearance of lesions [Table/Fig-5].
(a) Extensive lesions in a Patient (Acyclovir group); (b) 7th day follow-up (c) 14th day follow-up
(a) Few lesions at presentation (Placebo group); (b) 7th day follow-up: Appearance of new lesions
Response to treatment in acyclovir and placebo groups
| State of skin lesions | No. of patients (acyclovir group) | No. of patients (placebo group) | p-value |
|---|
| Regression on 7th day | 16 | 3 | p=0.0003* |
| Regression on 14th day | 26 | 10 | p=0.0001* |
| Unchanged on 7th day | 3 | 17 | p=0.0001* |
| Unchanged on 14th day | 0 | 4 | p=0.0384* |
*-p<0.05-statistically significant as determined by z-test. The results are for 30 patients each in the acyclovir and placebo group.
Appearance of new lesions in acyclovir and placebo groups
| Day | No of patients (acyclovir group) | No. of patients (placebo group) |
|---|
| 7th day | 0 | 3 |
| 14th day | 1 | 2 |
Time taken for clearance of skin lesions in acyclovir group (<7 days vs. >7 days)
| Acyclovir | Treatment started <7 days after nset of lesions | Treatment started >7 Days after onset of lesions | p-value |
|---|
| No. of days taken to clear | 5.33 | 6.65 | p=0.2874* |
*p>0.05- no statistical significance as determined by student’s t-test
Discussion
Pityriasis rosea is an acute papulo-squamous disorder of unknown aetiology. It is a self-limiting condition and usually treated symptomatically. Seasonal occurrence and clustering of cases led to the presumption of an infectious aetiology in pityriasis rosea and some antimicrobials have been tried accordingly. The course of the disease resembles viral exanthema. In some patients, history of prodromal symptoms and recent upper respiratory tract infection is present [11,21]. Spontaneous resolution of the lesions is also consistent with a viral aetiology [11]. Recent studies have focused on an association of pityriasis rosea with HHV-6 and HHV-7 [17]. DNA of HHV-6 and HHV-7 was found in lesional (86% and 93%) and non-lesional skin (79% and 86%), saliva (80% and 100%), peripheral blood mononuclear cells (83% in case of both) and serum (88% and 100%) of patients with pityriasis rosea by polymerase chain reaction [16].
The pathogenetic mechanism for the majority of the viral exanthems is still unclear. It has been proposed that the virions invade the extravascular dermal spaces from the blood vessels and damage the dermal or epidermal tissues either directly or by their interaction with the host immune system [22]. There is no evidence of active infection by Cytomegalovirus, Epstein-Barr virus and Parvovirus B19 in PR [23].
Presumably the productive infection arises from reactivation, since these patients most likely have been infected with both of these viruses previously during childhood. There is also a possibility of interaction between HHV-6 and HHV-7. In-vitro experiments have demonstrated that HHV-7, but not HHV-6, may be reactivated from latently infected blood mononuclear cells by T-cell activation [24]. However, latent HHV-6 could be recovered when co-infection with HHV-7 was established, suggesting HHV-7 can provide a transacting function allowing the reactivation of latent HHV-6 [24]. It has also been shown that reactivation of one virus may occur with primary infection of the other. Most frequently, primary HHV-7 appears to elicit HHV-6 reactivation, although the opposite may also occur. So, pityriasis rosea is not caused by direct herpes virus infection of the skin, but probably by cutaneous infiltration of latently infected lymphocytes during systemic viral replication [11]. Electron microscopy of lesional skin has also shown large numbers of virions resembling human herpes viruses in collagen fibers and blood vessels of upper and mid dermis [22].
Various treatment modalities have been tried in pityriasis rosea. Sharma et al., [9] had conducted a double blind, placebo-controlled trial using oral erythromycin stearate in adults (250 mg q.i.d. X 2 weeks) and children (25 to 40 mg q.i.d.) suffering from pityriasis rosea. Total 33 patients in the treatment group achieved complete response within 2 weeks of treatment with erythromycin compared to none in the placebo group [9]. UVB phototherapy was found to have resulted in decreased severity of pityriasis rosea in a bilateral comparison study [25]. A case of severe vesicular pityriasis rosea had been treated successfully with dapsone [26]. In view of probable viral aetiology, anti-viral drugs may be effective in pityriasis rosea. Ganciclovir, foscarnet, and cidofovir are active against HHV-6, but these agents have serious side effects like myelosuppression and nephrotoxicity. Acyclovir, in high dosage, has been proved to be effective against HHV-6 [17]. HHV-7 is less sensitive to acyclovir as it lacks the thymidine kinase gene, upon which the action of acyclovir is dependent [27]. However acyclovir is an easily available drug with lower side effects. Drago et al., [17] conducted a placebo-controlled trial with high dose of oral acyclovir (800 mg 5 times daily X 7 days) in patients with pityriasis rosea. Following this therapy, complete and partial remissions were observed within 2 weeks in 30 % and 62% patients respectively. Lesions in the acyclovir treated group cleared in significantly shorter time compared with the placebo group. Alleviation of the systemic symptoms after 7 and 14 days was better in the acyclovir treated group than in the control group [17]. A similar study done by Rassai et al., showed reduction in erythema among 46.4% and 78.5% patients receiving acyclovir at the end of first and second week respectively, even though they used a lower dose of acyclovir (400 mg five times a day) [18]. Ehsani et al., [19] compared the efficacy of acyclovir and erythromycin in pityriasis rosea. They had a similar follow-up assessment methodology as by Drago et al., [17] and the present study; however none of the patients, either in the acyclovir or in the erythromycin group showed complete response at the end of two weeks. They obtained a statistically significant result only at the end of eight weeks.
The results of the present study showed that significantly higher number of patients had complete regression of skin lesions in the acyclovir group compared to the placebo group on 7th and 14th days after the first visit. Greater number (p<0.05) of patients in the placebo group failed to show any improvement compared to the acyclovir group both on the 7th and 14th days. The response achieved on the 7th day is more important, because in the course of the disease, a spontaneous remission is unlikely at that point of time. These observations suggest that administration of acyclovir resulted in faster resolution of skin lesions in pityriasis rosea. A comparison of results with the study conducted by Drago et al., [17] and Rasai et al., [18] in relation to clinical response (complete response / no response) have been presented in [Table/Fig-6]. The present study showed a higher rate of complete response compared to the above-quoted studies. There was no significant difference in the time taken for resolution of skin lesions in the acyclovir group, whether the treatment was started within or after 7 days of onset. This finding is in concordance with the findings of Drago et al., [17] and led to the conclusion that response to acyclovir in pityriasis rosea was irrespective of the duration of illness. This is in contrast to other herpes virus infections where early administration of the anti-viral agent is necessary to achieve beneficial effects. The present study was randomized and double-blinded, with a better objectivity than the study by Drago et al., [17] which was non-randomized and single-blinded.
Comparison of results; complete response
| Trials | Acyclovir group | Placebo group |
|---|
| 7th day | 14th day | 7th day | 14th day |
|---|
| Drago et al., [17] | 28.6% | 78.6% | 4.5% | 4.4% |
| Rassai et al., [18] | 46.4% | 78.5% | 15.4% | 27% |
| Present study | 53.33% | 86.66% | 10% | 33.33% |
Conclusion
This is the first study assessing the efficacy of acyclovir in pityriasis rosea from India. From the results of this study, it may be concluded that high dose of acyclovir is effective in the treatment of pityriasis rosea and this efficacy is irrespective of the duration of the disease.
*-p<0.05-statistically significant as determined by z-test. The results are for 30 patients each in the acyclovir and placebo group.
*p>0.05- no statistical significance as determined by student’s t-test