Brown’s tumour is an uncommon focal giant cell lesion which arises as a result of the effect of increased parathyroid hormone on bone tissues in hyperparathyroidism. The mandible is the predominantly affected site in the maxillofacial area and a maxillary involvement is rare. The severity of the lesion, caused by a Brown’s tumour, may lead to evident osteolysis and gross deformity in the maxillofacial region, which suggests the need for making an early diagnosis and giving prompt treatment. We are reporting a male patient who presented with a massive painful swelling in the right maxilla as the first manifestation of primary hyperparathyroidism, caused by a parathyroid adenoma.
Brown’s tumour, Hyperparathyroidism, Parathyroid adenoma
Case Report
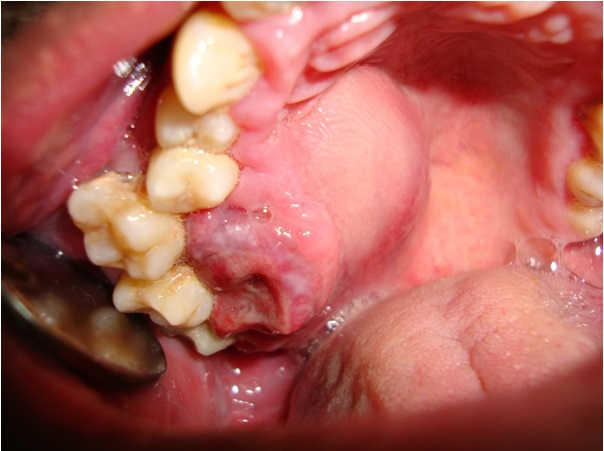
A 34-year-old male patient reported to the Department of Oral Medicine and Radiology, Government Dental College and Research Institute, Bangalore India, with the chief complaint of a swelling on the right side of the face of 4 months duration and pain of 2 months duration. The patient had difficulty in chewing food since 4 months, after which he had noticed a swelling on the right side of the face, which was insidious in onset, initially small in size and had gradually progressed to the present size. His medical history revealed that he suffered from epilepsy since 25 years and that he was under medication. On physical examination, the patient was found to be short statured and his vital signs were in normal range. Extraoral examination revealed a diffuse swelling on the right side of the face, which led to gross asymmetry and the skin over the swelling appeared to be unaltered. On palpation, the swelling was found to be tender and firm in consistency. The submandibular lymph nodes on the right side of the face were inflammed, tender on palpation and mobile. On intraoral examination, a diffuse swelling was seen on the right side of the hard palate, which roughly measured about 4 x 3 cm, with anterior extension from the distal aspect of the canine, posteriorly to the tuberosity area, which caused buccal displacement of the maxillary molar teeth and Grade III mobility, which caused hindrance in speech and mastication. The mucosa present over the swelling appeared to be inflamed, with indentation of the lower molar teeth [Table/Fig-1]. On palpation, the swelling was found to be tender and soft in consistency. The patient indicated that these lesions had been present since about 1 year, but that they had rapidly enlarged in size since 4 months. Based on the clinical findings, a provisional diagnosis of a benign soft tissue neoplasm was made, because of the site of occurrence, history and character. On routine radiographic examination, IOPA of maxillary molars and orthopantamograph revealed a generalized loss of lamina dura around teeth, with change in occlusal plane and a diffuse radiolucency above the apex of right maxillary molar teeth, which were suggestive of bony destruction [Table/Fig-2,3and4]. A radiographic differential diagnosis of primary hyperparathyroidism, osteomalacia and Paget’s disease of bone was given. Computed tomography (CT) scan of head and neck which was done, revealed a well defined irregular heterogenous soft tissue attenuation, which measured 5.4 x 5.9 x 5.2 cm, which was an osteolytic, bone-expanding lesion seen in the anterior part of the right maxillary sinus, with invasion of the adjacent floor of the orbit, anterior ethmoids and nasal cavity. Post contrast studies showed an early intense enhancement with few non enhancing areas within the lesion [Table/Fig-5,6and7]. The laboratory findings were as follows: intact PTH 314 pg ml-1 (15–65 pg ml-1), calcium 12.0 mg dl-1 (8.5–10.5 mg dl-1), phosphate 6.7 mg dl-1 (2.7– 4.5 mg dl-1) and alkaline phosphatase 283 U L-1.
A diffuse swelling present in the right palatal region, with indentation of the lower molar teeth and buccal displacement of maxillary molar teeth
IOPA in the region of 13,14 shows loss of lamina dura
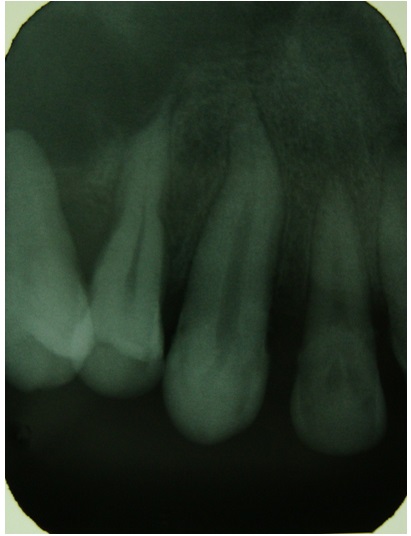
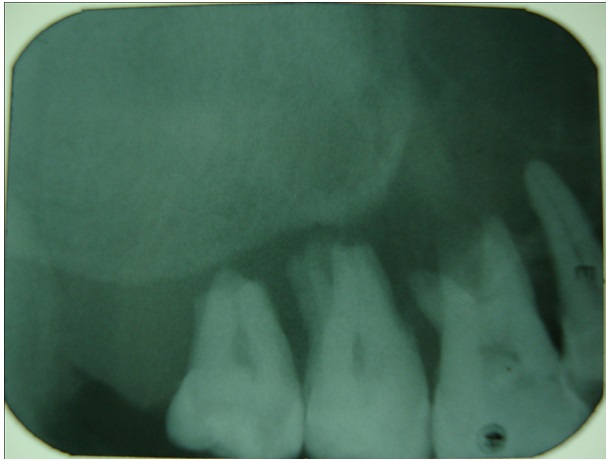
IOPA in the region of 16,17,18 shows loss of lamina dura and widening of periodontal ligament space
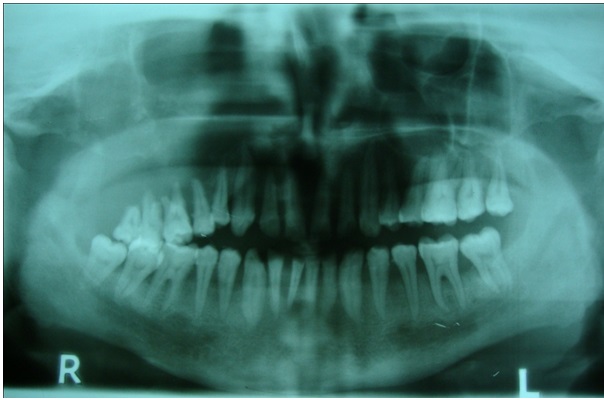
Orthopantomograph reveals loss of lamina dura around teeth and a change in the occlusal cant
Axial CT scan revealing a exophytic mass in the maxilla involving the medial wall of nose and floor of orbit (without contrast)
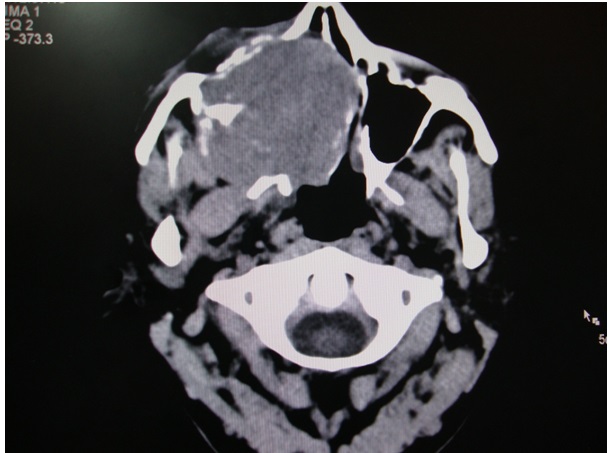
Axial CT scan revealing a exophytic mass in the maxilla involving the medial wall of nose and floor of orbit (with contrast)
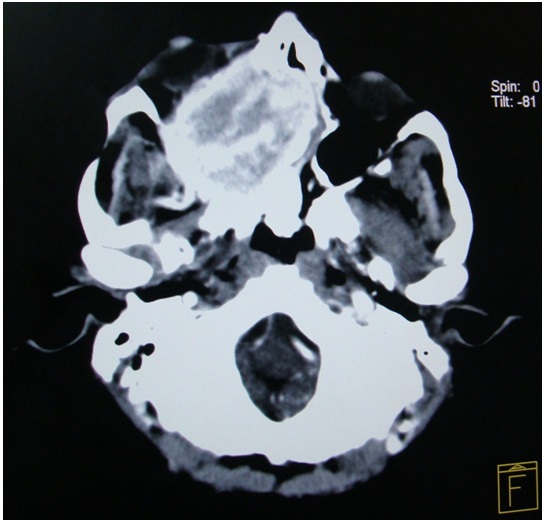
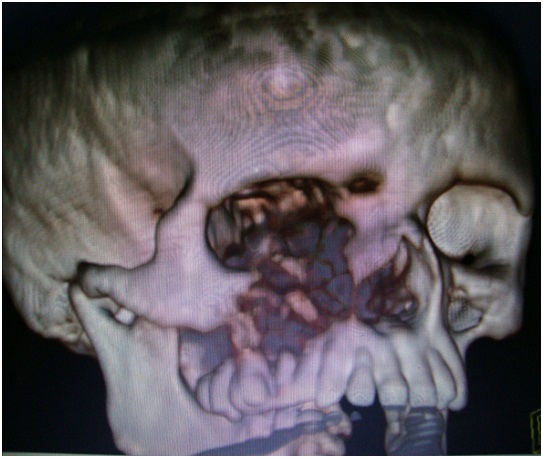
Coronal reformat and volume rendered image showing a round, bone-expanding lesion in the anterior part of the right maxillary sinus with invasion of the adjacent floor of the orbit, anterior ethmoids and nasal cavity
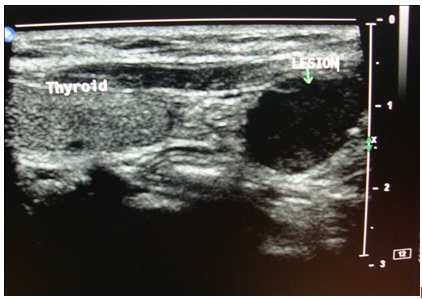
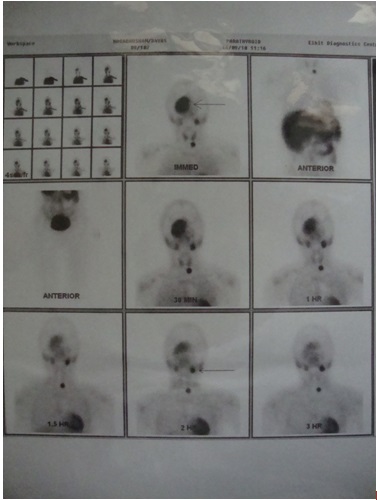
Ultrasonogram (USG) of the neck showed a well defined, homogenous, hypoechoic circular lesion which measured 1.5 x 2 cm, which was noted to lie inferior to the lower pole of left lobe of thyroid [Table/Fig-8]. The lesion was placed medial to the mid left common carotid artery and lateral to the cervical oesophagus, just deep in the subcutaneous plane, which showed minimal internal vascularity and was suggestive of a parathyroid adenoma. There was no evidence of significantly enlarged cervical lymph nodes . The thyroid gland appeared to be normal in size, shape and location. Ultrasound guided fine needle aspiration cytology (FNAC) revealed on microscopic examination; many multinucleated giant cells which were arranged in groups, which were adjacent to haemosiderin granules within a fibrovascular haemorrhagic stroma. These findings were compatible with a diagnosis of Brown’s tumour of the maxilla, which was associated with a parathyroid adenoma. Finally, to locate and define the extent of the spread of the tumour, a parathyroid Scintiscan was done after giving an IV injection of technetium-99m sestamibi, which showed a focus of abnormal accumulation of tracer in the location of the left inferior parathyroid gland, left submandibular region and right maxillary region [Table/Fig-9]. It was interpreted as a parathyroid adenoma which involved the left inferior parathyroid gland. Finally, after a thorough diagnostic work up, a diagnosis of Brown’s tumour of maxilla which was associated with primary hyperthyroidism was given.
Ultrasonography shows a hypoechoic lesion measuring 1.5 x 2 cm inferior to the lower pole of left lobe of thyroid
Technetium sestamibi bone scanning shows increased uptake in the inferior parathyroid and right side maxilla
Discussion
Hyperparathyroidism (HPT) was identified concurrently in Europe and United States in the mid 1920s. This disorder was first described in the setting of severe bone disease which resulted in significant morbidity. Incidence of primary hyperparathyroidism (PHPT) is 2- 3/1000 in women and it is 1/1000 in men. The disease is most frequent in postmenopausal women during the sixth decade of life, although it is seen in all age groups. Among patients with PHPT, 80–85% develop the disease due to parathyroid adenoma, 15–20% develop it due to hyperplasia of the parathyroid gland, while in less than 0.5%, the cause is parathyroid carcinoma [1–3].
Brown’s tumours are rare, focal giant-cell lesions which arise secondary to both primary and secondary HPT and they have been reported to occur in 4.5% of patients with primary HPT and in 1.5 to 1.7% patients with secondary disease [4]. Some parathyroid adenomas and hyperplasias are familial (5% of cases), and others are a part of multiple type I, IIa, and IIb endocrine neoplasias [5]. The ribs, femur and pelvis are more commonly involved sites of Brown’s tumours [6]. This bony lesion of HPT is caused by increased circulating levels of PTH, which result in increased osteoclastic bone resorption, primarily in cortical bone [7]. This may explain as to why the mandible, a cortical bone, is the most commonly affected site, and why a maxillary involvement is less common in the maxillofacial area [8].
In some cases, blood calcium levels which are within normal limits may also be observed. Although many systems are affected in PHPT, the most pronounced alterations are observed in the bone. With new and more objective diagnostic PTH radio-immunoassay techniques, along with the successful treatment of the disease, bony complications of HPT have started to decline rapidly. The earliest and most reliable change which is seen in hyperparathyroidism is subtle erosion of bone on the subperiosteal surfaces of the phalanges [9]. The lesion usually presents as a slight swelling in the jaw bones. However, our case was similar to the case which was presented by Dinkar et al., [6] and this case differed from previously reported cases with respect to its gross maxillary destruction. Radiographically, Brown’s tumour appears as a well defined unilocular or multilocular radiolucency and it is very similar to the other giant cell lesions like cherubism and aneurysmal bone cysts [10]. On clinical examination and on using only routine panoramic radiography, the lesions may resemble bone metastases of a carcinoma, multiple myeloma, Langerhan’s cell histiocytosis, osteosarcoma, Paget’s disease, osteomyelitis or osteonecrosis which is secondary to bisphosphonate therapy [8].
As it is difficult to distinguish Brown’s tumours from other giant cell lesions on the basis of histopathological and radiological examinations, the confirmatory diagnosis is made on the basis of hyperparathyroidism [11]. Brown’s tumours mainly result from secondary HPT in patients with renal insufficiency, but they have also been described as a rare manifestation of calcium malabsorption and some forms of osteomalacia [12]. Primary treatment of Brown’s tumour is surgical removal of associated parathyroid pathology. Brown’s tumours generally regress spontaneously over time and other treatment options include enucleation and curettage, radical resection and reconstruction, radiation therapy, and chemotherapy [13,14]. The most significant point about the case which has been described here, was the appearance of Brown’s tumours in the maxilla, which is rare and that the characteristic biochemical hallmark of primary hyper-parathyroidism was not seen. Thus, the patient whom we are describing to have normocalcaemic primary hyperparathyroidism was distinct from primary hyperparathyroidism patients who present with overt hypercalcaemia, in whom the serum calcium levels can occasionally be normal. From time to time, the serum calcium levels may be normal and most of the time, the serum calcium levels in these patients are elevated. Our case was similar to the case which was described by Bilizikian and Silverberg [15]. Maruani and Cols postulated that in normocalcaemic primary hyperparathyroidism, there was resistance to target tissues. After an oral calcium load, normocalcaemic subjects showed inadequate suppression of PTH when they were matched with a cohort of subjects who had hypercalcaemic primary hyperparathyroidism [16]. Surgical excisions of Brown’s tumours are indicated for large and disfiguring lesions and in cases where the affected bone is weakened, as it was seen in our case. However, other authors had initially treated such lesions with systemic corticosteroids and, when they had reduced in size, they had done surgical excisions [17]. The present report describes a rare case of a peripheral Brown’s tumour which was associated with primary HPT, which simulated a peripheral giant cell lesion of the jaws.
Conclusion
This case emphasizes the importance of making an early diagnosis of hyperparathyroidism by doing a thorough diagnostic work-up. This case should attract the attention of general practitioner dentists, oral and maxillofacial surgeons, endocrinologists and radiologists, whose consultations may be vital for patients with hyperparathyroidism, since this disease may result in nearly fatal results if it is neglected. Dentists should also be alert to the possible occurrence of Brown’s tumours in the jaws of patients who had previously been diagnosed to have hyperparathyroidism.
[1]. Guimaraes LS, Silva LM, Gomes CC, Castro WH, Mesquita RA, Gomez RS, Peripheral Brown’s tumour of hyperparathyroidism in the oral cavityOral Oncology Extra 2006 42:91-3. [Google Scholar]
[2]. Ahmad R, Hammond JM, Primary, secondary, and tertiary hyperparathyroidismOtolaryngol Clin North Am 2004 37:701-13. [Google Scholar]
[3]. Mantar F, Gunduz S, Gunduz UR, A reference finding rarely seen in primary hyperparathyroidism: Brown’s tumorCase Rep Med 2012 2012:432676 [Google Scholar]
[4]. Proimos E, Chimona TS, Tamiolakis D, Tzanakakis MG, Papadakis Brown’s tumor of the maxillary sinus in a patient with primary hyperparathyroidism: a case reportCEJ Med Case Rep 2009 3:7495 [Google Scholar]
[5]. Fitzgerald P, EndocrinologyIn Current Medical Diagnosis and Treatment 2000 39th editionStamford, CTAppleton and Lange:1118-21.Edited by Tierney LM, McPhee SJ, Papadakis MA [Google Scholar]
[6]. Dinkar AD, Sahai S, Sharma M, Primary hyperparathyroidism presenting as an exophytic mandibular massDentomaxillofac Radiol 2007 36:360-3. [Google Scholar]
[7]. Daniels JS, Primary hyperparathyroidism presenting as a palatal Brown’s tumorOral Surg Oral Med Oral Pathol Oral Radiol Endod 2004 98:409-13. [Google Scholar]
[8]. Selvi F, Cakarer S, Tanakol R, Guler SD, Keskin C, Brown’s tumour of the maxilla and mandible:a rare complication of tertiary hyperparathyroidismDentomaxillofac Radiol 2009 38(1):53-8. [Google Scholar]
[9]. Kar DK, Gupta SK, Agarwal A, Mishra SK, Brown’s tumor of the palate and mandible in association with primary hyperparathyroidismJ Oral Maxillofac Surg 2001 59:1352-4. [Google Scholar]
[10]. Nair PP, Gharote HP, Thomas S, Guruprasad R, Singh N, Brown’s tumour of the jawBMJ Case Reports 2011 :1-4. [Google Scholar]
[11]. Mirra JM, Bone tumorClinical, Radiologic, and Pathologic Correlations 1989 Philadelphia, PALea and Febiger:1785 [Google Scholar]
[12]. Dusunsel R, Guney E, Gunduz Z, Maxillary Brown’s tumor caused by secondary hyperparathyroidism in a boyPediatr Nephrol 2000 14:529-31. [Google Scholar]
[13]. Vikram HR, Petito A, Bower BF, Goldberg MH, Parathyroid carcinoma diagnosed on the basis of a giant cell lesion of the maxillaJ Oral Maxillofac Surg 2000 58:567-9. [Google Scholar]
[14]. Alvarado F, Waguespack SG, Campbell JH, Williams TP, Expansile intraosseus lesion of the mandibleJ Oral Maxillofac Surg 2003 61:1318-23. [Google Scholar]
[15]. John P, Bilezikian, Shonni J. Silverberg. Normocalcemic primary hyperparathyroidismArq Bras Endocrinol Meabol 2010 54:106-9. [Google Scholar]
[16]. Maruani G, Hertig A, Paillard M, Houillier P, Normocalcemic primary hyperparathyroidism: evidence for a generalized target-tissue resistance to parathyroid hormoneJ Clin Endocrinol Metab 2003 88:4641-8. [Google Scholar]
[17]. Martinez-Gavidia EM, Bagan JV, Milian-Masanet MA, Lloria de A, Perez-Valles A, Highly aggressive Brown’s tumour of the maxilla as first manifestation of primary hyperparathyroidismInt J Oral Maxillofac Surg 2000 29:447-9. [Google Scholar]