Mankind has made significant advances in healthcare. In India, longevity has doubled from 32 years in 1947 to 66 years [1]. With advances in the medical science we have been able to prolong the life span, but there has not been a similar improvement in the quality of life. One of the main problems undermining the quality of life in the geriatric population is a progressive cognitive decline. At present, of the 18 million people suffering from dementia worldwide, 60% belong to developing countries. More than 33% of women and 20% of men aged 65 and older will develop dementia during their lifetime. In 2008, the World Health Organization (WHO) declared ‘Dementia’ as a priority condition through the Mental Health Gap Action Programme [1].
The most common form of dementia, Alzheimer’s disease (AD), affects about 5.4 million people in the United States alone, and that number is projected to reach 12-16 million by the year 2050. In the United States in 2011, the cost of health care, long-term care, and hospice services for people aged 65 years and older with AD and other dementias were expected to be $183 billion [1]. It has been estimated that there will a 40% increase in AD in Europe, 63% increase in North America, a 117% growth in East Asia, 107% in South Asia and 125% in North Africa and the Middle East [2].
Cognitive neuropharmacology is still in its infancy. Use of cognitive drugs have been controversial and there is no general consensus on their efficacy in humans [3]. Cognition enhancers (CE), including cholinesterase inhibitors (for example, Donepezil, Galantamine and Rivastigmine) and Memantine (N-methyl-D-aspartic acid (NMDA) receptor antagonist) have been approved for the treatment of Alzheimer’s disease in many countries. Other drugs hypothesized to have a cognition enhancing effect inlcude: i) Nootropics – E.g Piracetam [4], ii) Noradrenergic and Dopaminergic system modulating drugs – E.g. Methylphenidate and Atomoxetine; iii) Caffeine and iv) Modafinil [5].
Cost concerns play an important role during CE drug therapy. Alzheimer’s disease drugs have limited availability and are unaffordable in low and middle income countries compared to high income countries in terms of Purchasing Power Parity [6].
Keeping in mind the extreme importance of managing Alzheimer’s and other cognitive disorders and the paucity of drug utilization research in dementia, we conducted a study with the following objectives:
Methodology
A prospective, cross sectional drug utilization study (DUS) was conducted after the Institutional Ethics Committee (IEC) approval. The ‘Strengthening the Reporting of Observational Studies in Epidemiology’ (STROBE) guidelines and the WHO recommendations on conducting DUS [7] were used in preparation of protocol and the manuscript [8]. One hundred prescriptions of patients of both sexes and all ages, suffering from dementia as diagnosed by the physicians based on the ICD – 10 criteria (International Classification of Diseases) and started on at least one CE drug, were selected after explaining to them the scope of our study and obtaining their written informed consent.
The study sites were the Neurology and Psychiatry outpatient department of tertiary care hospitals in Mumbai, and the study duration was from 1st July to 31st August 2011, The sampling frame was fixed at three prescriptions per day, five days a week for the first month and two prescriptions per day, five days a week for the second month, during the given sampling period.
The three/two prescriptions were selected as follows: On day 1, all prescriptions were chosen from the beginning of the day, on day 2 all prescriptions were chosen from the middle of the day and on day 3 all prescriptions were chosen from the end of the day and so on [7]. In case of OPD holidays or when the required numbers of prescriptions were not obtained, the prescriptions of that day were assigned to the next working day.
The data analysis and statistical evaluation was done using Microsoft ®Excel ® 2007 software version 12.0.4518.1014. The following data were collected:
a. Patient details like age, gender, education, occupation, monthly family income, registration number and diagnosis. Prescription details like number of drugs, and names of individual drugs (generic/brand), any Fixed Dose Combination (FDC) prescribed, dose, dosage form, dosing schedule, duration of treatment and availability of prescribed drug in the hospital pharmacy.
The updated Kuppuswamy’s scale was used for socioeconomic categorization of the patients [9]. The WHO-INRUD (International Network for the Rational Use of Drugs) drug use indicators were used to assess the observed prescription patterns [7]. The prescribed drugs were classified according to The Anatomical Therapeutic Chemical (ATC) – Defined Daily Dose (DDD) classification [10]. The prescribed daily dose (PDD) was calculated by taking the average of the daily doses of the CE drugs as the PDD. The PDD to DDD ratio was then calculated. For cost analysis, the cost of drugs was obtained from the hospital rate contract and/ or the Drug Index (DI): April – June 2012. For drugs prescribed from outside pharmacies we calculated the price per DDD (minimum and maximum), and the Cost Index (CI)
Results
The participant characteristics are as shown in [Table/Fig-1]. The mean age was 64.04 years with a range of 39 to 88 years. Out of 100, 44 patients were diagnosed with ‘Unspecified Dementia with Psychoses’, 26 with ‘Vascular Dementia’, 18 with ‘Alzheimer’s disease’, eight with ‘Unspecified Dementia with Depression’ and four with ‘Dementia in Parkinson’s disease’.
Characteristics of participants (n=100) suffering from various types of dementia and attending the psychiatry/ neurology outpatient department, Mumbai, 1st July to 31st August 2011
| Characteristic | Number of participants out of n = 100 |
|---|
| Age (years) | 35-45 | 4 |
| 46-55 | 24 |
| > 56 | 72 |
| Sex | Male | 84 |
| Female | 16 |
| Marital Status | Married | 76 |
| Unmarried | 4 |
| Widowed | 20 |
| Socio-economic status * | I | 12 |
| II | 0 |
| III | 32 |
| IV | 52 |
| V | 4 |
* As per the updated Kuppuswamy’s Socio-economic scale
Sixty four participants had comorbid disorder (s) like diabetes (24/64), cardiovascular disease (32/64), Chronic Obstructive Pulmonary disease (12/64), constipation (12/64), scabies (12/64) and benign prostatic hypertrophy (12/64). The analysis of prescription patterns as per the s WHO/ INRUD a drug use indicator is depicted in [Table/Fig-2]. All the prescriptions were replete in terms of the following essential components: the dose, dosage form, frequency and instructions for drug use. In all, the 100 prescriptions contained 322 drugs out of which, 168 were CE drugs. There was no prescription with more than 4 drugs. The various CE drugs prescribed are shown in [Table/Fig-3].
Assessment of the prescription pattern, as per various drug use indicators, in a sample of participants (n=100) suffering from various types of dementia and attending the psychiatry/ neurology outpatient department, Mumbai, 1st July to 31st August 2011
| Sr. No. | Drug use indicators | Result |
|---|
| 1. | Average number of drugs per prescription : Mean ± SD | 3.22 ± 0.12 |
| 2. | Average number of CE drugs per prescription : Mean ± SD | 1.68 ± 0.08 |
| 3. | Percentage of prescriptions containing CE FDCs | 0% |
| 4. | Percentage of drugs prescribed by generic name | 38.2% (123/322) |
| 6. | Percentage of prescriptions with an injection prescribed | 0% |
| 7. | Percentage of CE drugs prescribed from the hospital drug schedule | 0% |
Percent utilization of CE drugs in a sample of participants (n=100) suffering from dementia and attending the psychiatry/ neurology outpatient department, Mumbai, 1st July to 31st August 2011
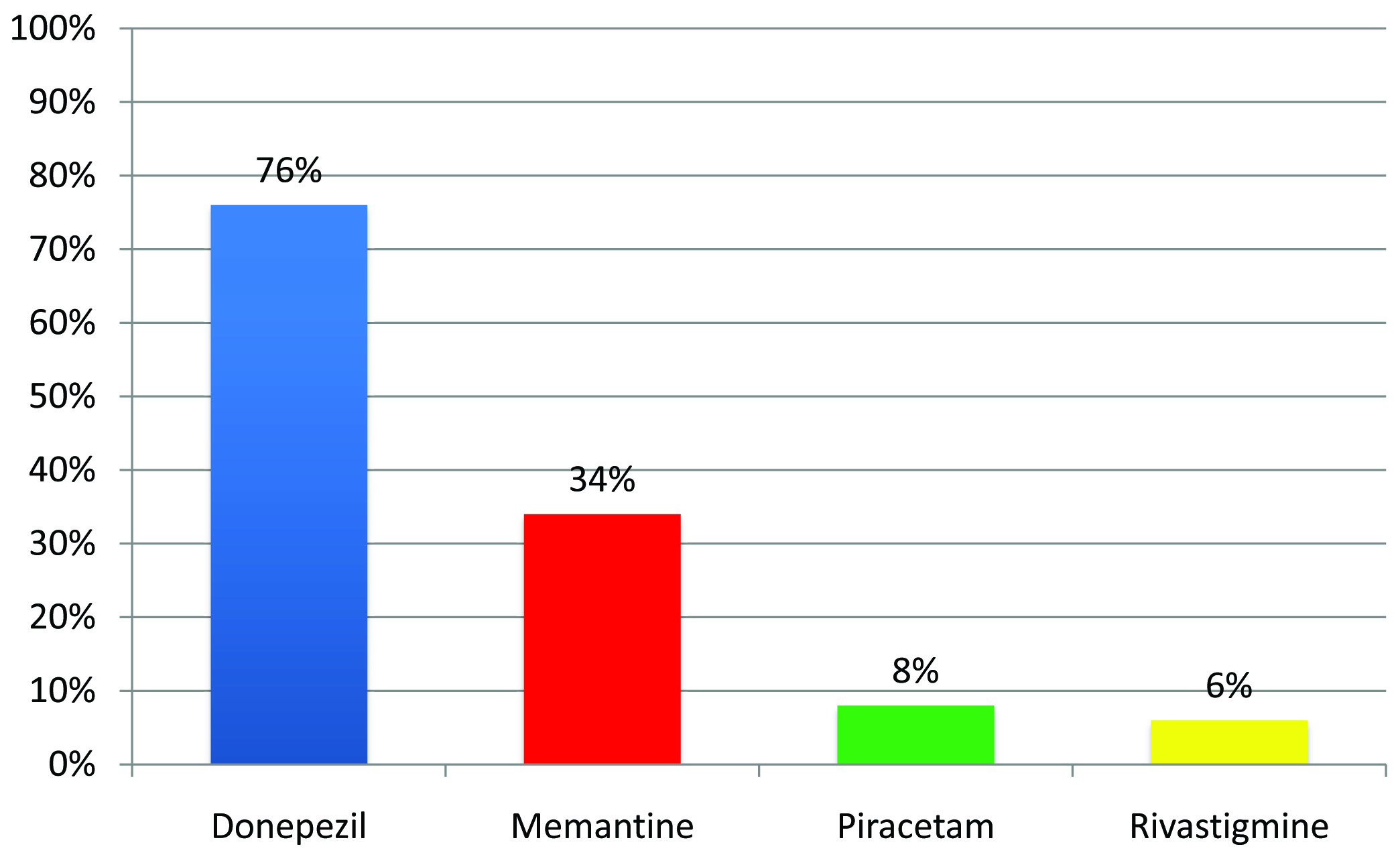
The other drugs commonly co-prescribed are shown in [Table/Fig-4]. There were no potential drug interactions among the drugs prescribed.
Percent utilization of drugs co-prescribed in a sample of participants (n=100) suffering from various types of dementia and attending the psychiatry/ neurology outpatient department, Mumbai, 1st July to 31st August 2011
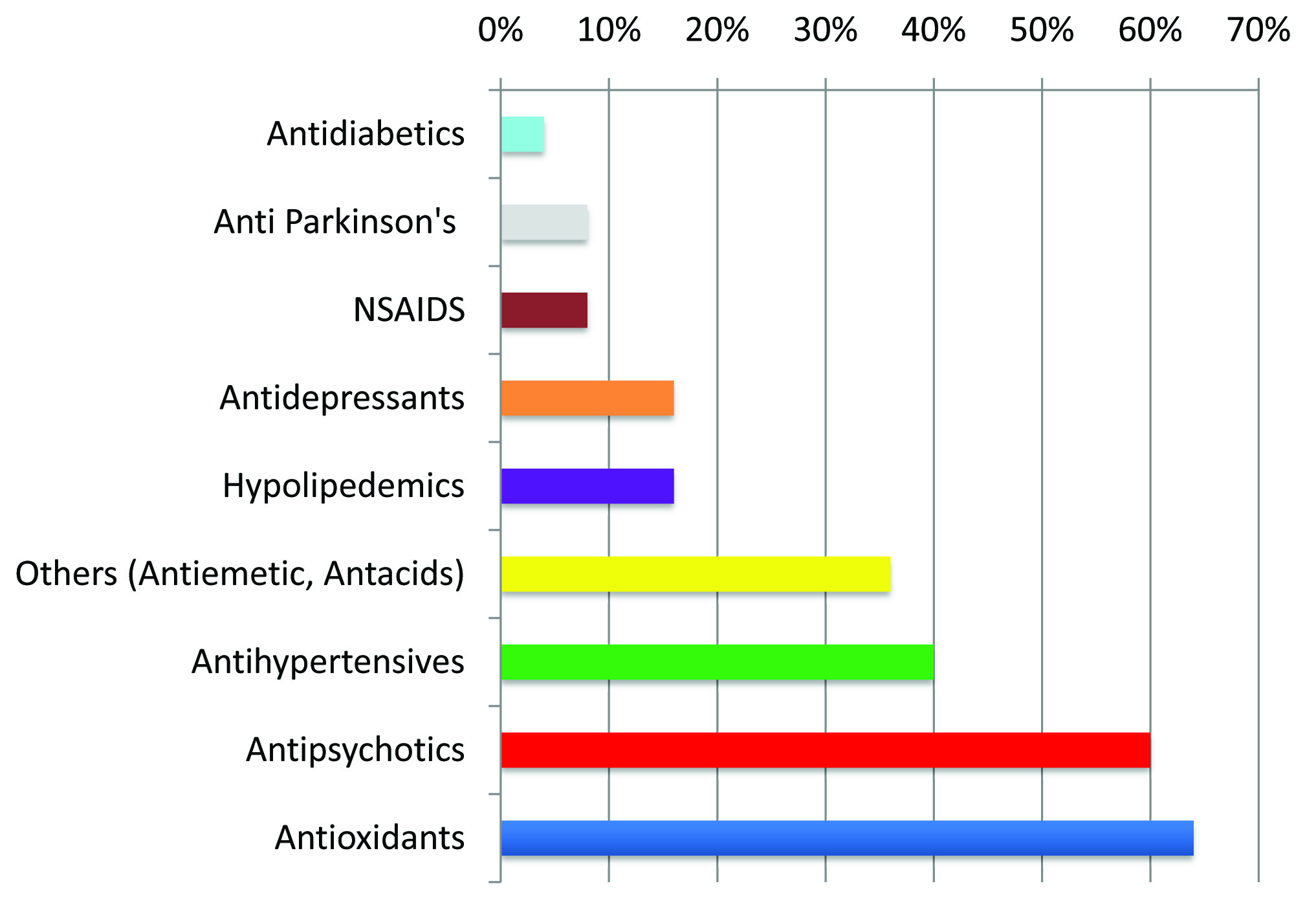
The pattern of CE drug use as per the ATC/DDD classification is shown in [Table/Fig 2–5]. The DDDs mentioned in the table are for the oral route as obtained from the WHO ATC/DDD website 2012 [11].
ATC/DDD classification, PDD values and PDD/DDD ratio of CE drugs prescribed to a sample of participants (n=100) suffering from various types of dementia and attending the psychiatry/ neurology outpatient department, Mumbai, 1st July to 31st August 2011
| S. No. | Drug | ATC code | DDD* (mg) | PDD (mg) | PDD/DDD |
|---|
| 1. | Donepezil | N06DA02 | 7.5 | 10.2 | 1.36 |
| 2. | Rivastigmine | N06DA03 | 9 | 6.5 | 0.72 |
| 3. | Memantine | N06DX01 | 20 | 18.75 | 0.94 |
| 4. | Piracetam | N06BX03 | 2400 | 1440 | 0.6 |
The average cost per prescription was 626.29 or USD 9.5, out of which 1334.44 (53.4%) was on CE drugs. The hospital bore 42.52% of the total cost. The CE drugs were analyzed further in terms of their cost as shown in [Table/Fig-6].
Cost analyses of CE drugs prescribed to a sample of participants (n=100) suffering from various types of dementia and attending the psychiatry/ neurology outpatient department, Mumbai, 1st July to 31st August 2011.
| S. No | Drugs | Price per DDD (₹) | Cost Index (b/a) |
|---|
| Min (a) | Max (b) |
|---|
| 1. | Donepezil | 9 | 22.9 | 2.5 |
| 2. | Rivastigmine | 18.4 | 34.5 | 1.9 |
| 3. | Memantine | 19.2 | 26 | 1.4 |
| 4. | Piracetam | 15 | 39.6 | 2.6 |
*The minimum and maximum cost was obtained from the Drug Index: April–June 2012 (13).
Discussion
Overall, the principles of rational prescribing were followed according to the various WHO/INRUD drug use indicators.
The most commonly prescribed drugs in our study were donepezil and memantine. The least commonly prescribed drugs were piracetam and rivastigmine, in that order. Similar results were also reported in other studies [12–14]. Galantamine was not prescribed to anyone.
The ‘American College of Physicians’ guidelines state that clinicians should base the decision to initiate therapy with a cholinesterase inhibitor or memantine on individualized assessment. They further say that one has to strike balance between harm and doubtful benefit of the CE drugs [15]. According to the National Institute of Health & Care Excellence (NICE) guidelines, donepezil, galantamine and rivastigmine are recommended as options for managing mild to moderate Alzheimer’s disease, and memantine is recommended for managing moderate to severe Alzheimer’s disease [16].
Piracetam is said to have antithrombotic, neuroprotective and rheological properties and has been advocated in various disorders like dementia, vertigo, myoclonus and stroke [17]. In a multicentre 12 month trial in mild cognitive impairment patients, it was found that there were no difference between piracetam and placebo [18]. Many other studies have also expressed doubts on the usefulness of piracetam [19–21]. Rivastigmine is the only CE drug US FDA approved for ‘Dementia in Parkinson’s disease’ [14]. In our study, there were only four participants diagnosed with ‘Dementia in Parkinson’s disease’ and hence, the low prescription of rivastigmine.
The most commonly co-prescribed drugs were antioxidants (AO). But evidence supporting their use is lacking [22]. The next most commonly co-prescribed drugs were antipsychotics, because the most commonly diagnosed type of dementia was ‘Unspecified Dementia with Psychoses’ and psychotic features may develop in the later stages of other forms of dementia also [23,24]. In cases of psychotic symptoms in geriatric dementia patients, drug therapy should be started only if required and should be governed by a “start low, go slow” paradigm with a single agent. Atypical antipsychotics have the greatest effectiveness and are best tolerated [24]. But there exist concerns regarding the use of atypical antipsychotics in elderly patients due to the increased risk of mortality. The US FDA has advocated a ‘Boxed Warning’ in their labeling describing this risk and noting that these drugs are not approved for this indication. The Agency is also considering adding a similar warning to the labeling for older antipsychotic medications [25]. The high prescription of antihypertensives and hypolipidemics can be explained by the high prevalence of hypertension and hyperlipidemia in the geriatric population. Antacids were also prescribed to a significant proportion. Antacids may affect the absorption of other medications and should not be prescribed in individuals with decreased renal function due to the risk of accumulation of aluminum and magnesium [26]. Long term Proton pump inhibitor therapy should also be avoided as, according to recent reports, they are known to cause osteoporosis, vitamin B 12 deficiency, interact with clopidogrel among other effects [27–29]. Thus, H2-Receptor antagonists should be preferably prescribed.
The PDD/DDD ratio of Memantine was closest to one. When the PDD/DDD ratio is either less than or greater than one, it may indicate either under or over utilization of drugs. But it is important to note that the PDD can vary according to ‘patient’ and ‘disease’ related factors. In addition, the DDDs obtained from the WHO ATC/DDD website are applicable for the management of ‘moderate’ intensity conditions and are based on international data. Thus, countries should have their own DDD values based on indigenous data.
Limitations
A limitation of all prospective observational studies is the Hawthornes bias, that is, the prescriber’s behavior might be influenced by the fact that they are being observed. A retrospective study can obviate this problem.
We didn’t evaluate efficacy and safety of the prescribed drugs (except for the potential drug interactions), as they were technically complex, time consuming and beyond the scope of our study.
Conclusion
The most common form of dementia was ‘Unspecified Dementia with Psychoses’ followed by Vascular Dementia and Alzheimer’s disease. The most commonly prescribed CE drugs were Donepezil and Memantine and hence they could be included in the Hospital Drug Schedule. The least commonly prescribed was Rivastigmine. Gallantamine was not prescribed at all. A major part of the total cost per prescription was borne by the patient as the CE drugs were not available in the hospital pharmacy.
Recommendations
Prescribing Piracetam should be avoided because of the high cost and doubtful benefits.
Antipsychotics should be used in geriatric dementia patients judiciously, due to an increase in mortality.
* As per the updated Kuppuswamy’s Socio-economic scale
*The minimum and maximum cost was obtained from the Drug Index: April–June 2012 (13).