Tubo-ovarian abscesses can rupture spontaneously after a manual examination or an accidental trauma. A critically ill patient with septic peritonitis will only deteriorate if timely surgical removal of pus is not done. The operation of choice is removal of free pus, together with the abscess, the uterus, the tubes and usually, the ovaries. Anatomy is distorted, dependable landmarks are obscured and tissues are thick and oedematous. Loops of densely adhered intestine are difficult to separate. If an intra peritoneal approach is used, it is likely that the fragments of ovary will be left behind. This can subsequently cause signs and symptoms of ovarian remnant syndrome. Injury to the serosa of distended bowel occurs inadvertently, thus increasing the morbidity which results from the procedure. We are hereby presenting a unique case of a ruptured tubo-ovarian abscess where a retroperitoneal approach was used.
Pelvic inflammatory disease, Retroperitoneal, Ruptured, Tubo-ovarian abscess
Case Report
A 46-year-old lady presented to the emergency department with complaints of pain in abdomen and loose stools of one day’s duration. She had high grade intermittent fever with chills for one day. She had parity 2, with a history of a tubal sterilization which had been done 20 years back. She had her last period fifteen days back and was febrile. There was mild pallor. Lower abdomen tenderness, rigidity and guarding were present. Right iliac fossae tenderness was present. On speculum examination, cervix was found to be unhealthy. On vaginal examination, the uterus was found to be of normal size, retroverted and slightly bulky. Right adnexal palpation revealed a tender mass of size, 5 x 6 cm. Her haemoglobin was 9 gm/dl and a peripheral smear study demonstrated neutrophilic leukocytosis.
Pelvic ultrasound showed a right adnexal mass of size, 69 x 73 x 58 cm, with free fluid. Uterine endometrium was thickened and uterine contour was indistinct. Ultrasound picture showed bowel shadows close to the mass. A pelvic CT was done to rule out the possibility of an appendicular abscess. CT scan picture showed loops of intestine which adhered to the right adnexal mass. Right ovary showed presence of gas shadows. Appendix could not be visualized separately. An emergency laparotomy was done and abdomen was opened in layers by making a midline vertical incision. Yellow flakes of pus were found in the peritoneal cavity, which were collected and sent for culture. Right tube and ovary formed an inflamed mass which discharged pus. Appendix and ileal loops were adhered. Uterus was erythematous. Left tube and ovaries also adhered to the lateral pelvic peritoneum. The planned objective of surgery was enucleating right tubo-ovarian mass, uterus, cervix and left tube and ovary away from the underlying peritoneum, by clamping the ligaments retroperitoneally. The rationale was to elevate the pelvic peritoneum as a false capsule around the bowel loops.
At first, an appendicectomy was done after adhesiolysis of appendix from bowel loops and tubo-ovarian mass. The colon was displaced medially to expose the sacral promontory and right side of the pelvic brim Lateral parietal peritoneum was incised at the lateral attachment of round ligament. Round ligament was identified, clamped and cut at the lateral pelvic wall. The medial and the lateral ends were held with silk, for them to act as retractors, for opening the retroperitoneal space [Table/Fig-1]. The peritoneal incision was extended posterior till the peritoneum, over the infundibulopelvic ligament. The peritoneum on the lateral side of infundibulopelvic was incised around the pelvic brim. The ureter was identified well above the pelvic brim. This provided a guide to the ovarian vessels which crossed the ureter at this level. The ovarian vessels were doubly ligated, some 7-8 cm from the ovary.
Tubo-ovarian mass is lifted and retroperitoneum opened with cutting the round ligament at the lateral pelvic wall
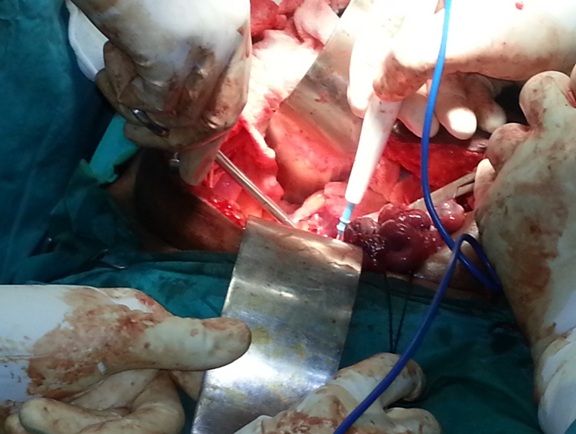
On the left side, the mesocolon was swept medially and the ureter was identified well above the pelvic brim. At level of pelvic brim, the left round ligament was clamped and cut. The medial and the lateral ends were held long to act as retractors. The peritoneum on the lateral side wall was then incised till the peritoneum which was present over the infundibulopelvic ligament. The ovarian vessels were doubly ligated and cut after identifying the ureter. The rectosigmoid was then elevated and two fingers were inserted into the retrorectal space. This is a relatively avascular space present in front of the presacral (Waldeyer’s) fascia and behind the superior haemorrhoidal vessels. The two paracolic dissections now communicated behind the rectosigmoid.
The uterovesical peritoneum was then incised and the bladder was stripped inferiorly. The areolar tissue was divided in the usual way to expose the cervix and upper vagina. The ureter was separated from the pelvic peritoneum. The lower one third of ureter was dissected medially, as it receives the blood supply from its lateral side (contrasting feature to upper two third). The ureteric dissection was continued downwards till the ureter entered the ureteric canal in the cardinal ligament. The tip of closed Moyniham’s gall bladder forceps was introduced on the roof of ureteric tunnel. The clamp was opened and the roof was divided. The same procedure was carried out on the other side. The uterine artery was then clamped and tied without causing any danger to the ureter. The bladder which was present below was further freed from vagina.
The assistant was asked to draw the fundus of uterus upwards and forwards, so that the pouch of Douglas was exposed. With non toothed forceps and dissecting scissors, the peritoneum on the pouch of Douglas was cut. The curved tip of the scissors was held away from bowel. The bowel was stripped away from the uterus and vagina in the rectovaginal space with swab holding forceps.
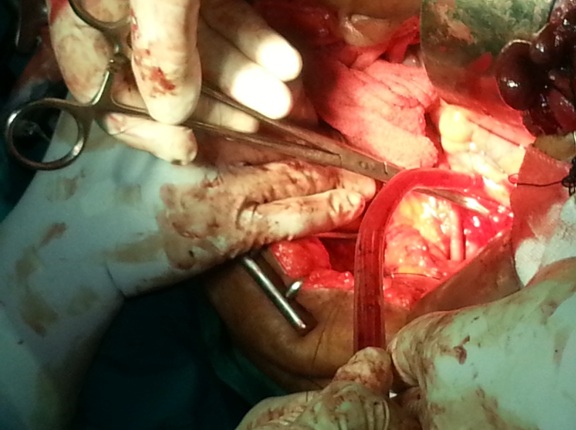
The fundus of the uterus was then drawn upwards and backwards and the cardinal ligaments were clamped and cut distally. Same procedure was done on the other side. Right angle clamps were applied on vagina and vaginal vault was transacted. The curved clamps were then placed on the uterosacral ligaments and tissues were cut through, distal to the clamps. The edges of vagina were grasped with long forceps. Peritoneal edges were checked for haemostasis. Thus, the inflamed tubo-ovarian mass was lifted, leaving behind the underlying peritoneum along with the adherent bowel; thereby avoiding injury to the bowel [Table/Fig-2]. Vaginal vault was left partially open. Haemostasis was secured. Reperitonization was not done. Peritoneal lavage was done by using normal saline. The abdomen was closed in layers.
Ligation of right uterine artery at its origin from the anterior division of internal iliac artery
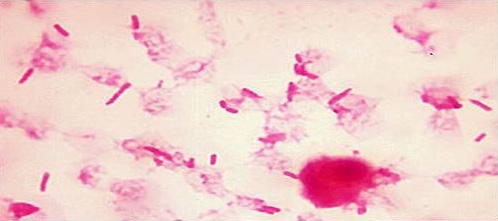
Post-operatively, the bowel sounds were sluggish. A possibility of post-operative paralytic ileus was speculated. The patient was kept nil orally for five days with intravenous fluids and Ryle’s tube aspiration She received a broad spectrum antimicrobial coverage with cefperazone sulbactum, clindamycin and amikacin parenterally. Three units of fresh whole blood were transfused on alternate days in the post-operative period, as the post-operative haemoglobin level was low. The patient also received antithromboses prophylaxis as enoxaparin 40 mg, subcutaneously twice daily for three days. A continuous bladder drainage was maintained for ten days. Stitch removal was done on post-operative day 10 and patient was discharged on post-operative day 21. Pus culture report demonstrated Escherichia coli, a Gram negative coliform which was sensitive to Imipenem, Piperacillin tazobactam, Cefoperazone sulbactam, Amikacin and Gentamicin and resistant to Ampicillin, Ceftazidime, Cefotaxime, Ciprofloxacin, and Cotrimoxazole, ([Table/Fig-3]. Gram staining showed Gram negative bacilli along with pus cells- Escherichia coli).
Gram Stain Picture sowing Gram Negative Bacilli along with pus cells - Microbiological study of the tubo-ovarian mass
Discussion
The term, ‘Pelvic Inflammatory Disease’ (PID) was traditionally used for inflammatory diseases of the parametrium, but in recent years, it has become clear that this term is being applied to diseases of the upper genital tract, those which occur above the cervical external os [1]. A pocket of pus that forms during infection of a fallopian tube and ovary is called as a tubo-ovarian abscess. Tubo-ovarian abscesses most commonly occur in women of reproductive age group. Acute or chronic upper genital tract infections are the primary cause in most of the cases. Rarely, endogenous organisms from small intestine or appendix can primarily or secondarily infect a tubo-ovarian mass. Symptomatic or subclinical infections can rapidly lead to tubo-ovarian abscesses. Untreated tubo-ovarian abscesses can rupture and they can lead to life threatening peritonitis [2]. In all cases of PID, further diagnostic tests or laparoscopy should be considered if symptoms do not improve or worsen after three days of medical treatment. This will prevent adhesions, ectopic pregnancies, inflammation of fallopian tubes and tubo-ovarian abscesses.
A tubo-ovarian abscess is usually diagnosed by a physical examination or pelvic ultrasound. Sometimes, an abscess is diagnosed only after a surgical exploration.Abscess drainage may be done by using a large bore, CT or USG guided needle. The abscess cavity can also be drained by laparoscopy or laparotomy. The transperitoneal approach is usually applied, although Te Linde’s Text Book of Operative Gynaecology mentions the retroperitoneal approach.
The reported annual incidence of pelvic inflammatory diseases in women who are aged 15-39 years is 10-13 per 1000 women [3]. The exact incidence of pelvic inflammatory diseases is unclear, because they cannot be diagnosed reliably, based on clinical symptoms and signs. As compared to laparoscopy, the positive predictive value of clinical diagnosis is only 65-90 % [2]. Sometimes, the infected tubes and ovaries may also have to be removed surgically [4]. Observational studies have suggested that delaying treatment by three days can impair fertility [5].
When the abdomen is first opened, the surgeon should explore it systematically. Retro peritoneal plane of dissection was used to prevent bowel injuries in our patient.The technique of retroperitoneal dissection is originally reserved for radical dissection in pelvic malignancies. Te linde’s Textbook of Operative Gynaecology mentions this approach for treatment of acute pelvic inflammatory disease, although modern gynaecologists do not apply it [6]. Retroperitoneal dissection leaves the bowel intact without injuring it, as the peritoneum is lifted along with the inflamed bowel, which acts as a pseudo capsule around it. An alternative approach is transperitoneal approach of sharp dissection of bowel. This can cause multiple bowel injuries and increased blood loss. It may also lead to incomplete removal of uterine adnexa. Even in the surgical practice of benign pathologies, the surgeon should learn the ureteric course and the retroperitoneal anatomy and get familiarized with the structures. This will ensure complete clearance in cases of tubo-ovarian abscesses, endometriosis, frozen pelvis, as in chronic pelvic inflammatory disease and tuberculosis. Retrograde hysterectomy technique also helps in entering the anterior fornix and circumcising the cervix first, before dividing the uterine arteries and parametrium. A volsellum is applied to the cervix, which is pulled up, thus making the division of uterine artery easier, without causing any hazard to the ureter [7]. Radical pelvic surgeries should always be combined with antithrombotic prophylaxis. Large pelvic iliac veins drain directly to inferior vena cava, which can spread the microthrombi directly into pulmonary vasculature after passing through the right side of heart. Low molecular weight heparin also acts as a protection against disseminated intravascular coagulation. The features of post-operative paralytic ileus should be identified early and they should be managed aggressively. Neuropraxia of urinary bladder and ureter can be managed by giving bladder rest by providing continuous urethral catheterization for ten days.
Conclusion
An enthusiastic team work which is undertaken, by making an early radiological diagnosis and doing an emergency laparotomy and microbiological studies, was rewarding. Vigilant post-operative care can detect a paralytic, post-operative ileus at the earliest. The technique of retroperitoneal dissection is still novel for acute pelvic inflammatory disease, although there are many advantages.
Learning Points
In women with acute abdomen, a diagnosis of pelvic inflammatory disease with a ruptured tubo-ovarian abscess should be considered.
An early CT scan can be useful for identifying an adjacent adhered bowel or appendix and it can give a clue about gas forming organisms.
Retroperitoneal dissection leaves the bowel intact without injuring it, as the peritoneum is lifted along with the inflamed bowel, which acts as a pseudo capsule around it.
An alternative approach is transperitoneal approach of sharp dissection of bowel. This can cause multiple bowel injuries and increased blood loss. It may also lead to incomplete removal of uterine adnexa.
Even in the surgical practice of benign pathologies, the surgeons should learn the ureteric course and the retroperitoneal anatomy and get familiarized with the structures. This will ensure complete clearance in cases of tubo-ovarian abscesses, endometriosis, frozen pelvis, as in chronic pelvic inflammatory disease and tuberculosis.
In retroperitoneal approach, the retrograde hysterectomy technique also helps in entering the anterior fornix and circumcising the cervix first, before dividing the uterine arteries and parametrium. A volsellum is applied to the cervix, which is pulled up, thus making the division of uterine artery easier, without causing any hazard to the ureter.
Radical pelvic surgeries should always be combined with antithrombotic prophylaxis. Antithrombotic prophylaxis can prevent deep vein thrombosis and stage of coagulation in disseminated intravascular coagulation.
[1]. Robinson Nicola, Beral Valerie, Ashley John SA, Trends in pelvic inflammatory diseases in England and WalesJournal of Epidemiology and Community Health 1981 35:265-70. [Google Scholar]
[2]. Samson Chandra, Role of laparoscopy in the management of pelvic inflammatory disease/ Tubo ovarian abscess compared to other modalitiesWorld laparoscopy hospital website [Google Scholar]
[3]. Simms I, Stephenson JM, Pelvic inflammatory disease epidemiology: what do we know and what we need to know?Sex Transm Infect 2000 76:80-7. [Google Scholar]
[4]. Mi Young Kim, Sung Eun Rha, Soon Nam Oh, Seung Eun Jung, Young Joon Lee, You Sung Kim, MR imaging findings of hydrosalpinx: A Comprehensive ReviewRadio graphics 2009 29:495-507. [Google Scholar]
[5]. Ross Jonathan, Pelvic inflammatory DiseaseBMJ Clinical evidence 2001 322:658-9. [Google Scholar]
[6]. Martens Mark G, te Lind’s Operative Gynaecology (9ed) 2003 PhiladelphiaLippincott Williams and Wilkins:28 [Google Scholar]
[7]. Hudson Christopher N, Marcus E, Setchell Shaw’s Textbook of Operative Gynaecology 2004 6th edIndiaElsevier:22 [Google Scholar]