Comparative Evaluation of Four Phenotypic Tests for Detection of Metallo-β-Lactamase and Carbapenemase Production in Acinetobacter baumannii
Aparna Shivaprasad1, Beena Antony2, Poornima Shenoy3
1 Assisstant Professor, Department of Microbiology, Father Muller Medical College, Mangalore, Karnataka, India.
2 Professor, Department of Microbiology, Father Muller Medical College, Mangalore, Karnataka, India.
3 Professor, Department of Microbiology, Father Muller Medical College, Mangalore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Beena Antony, Assisstant Professor, Department of Microbiology, Father Muller Medical College, Mangalore, Karnataka-575002, South, India.
Phone: +919448024684,
E-mail: beenafmmc@gmail.com
Introduction:Acinetobacter baumannii is an emerging multi-drug resistant opportunistic pathogen that causes a variety of nosocomial infections. In recent years, carbapenem resistance in A.baumannii has increased due to Ambler class B Metallo β-lactamases or class D OXA Carbapenemases.
Objective: The present study was undertaken to detect and compare the various phenotypic methods for MBL production in nosocomial A.baumannii isolates.
Materials and Methods: One hundred sixty eight A.baumannii isolates were subjected to disc diffusion assay. Imipenem resistant isolates were subjected to 4 different phenotypic tests. MBL screening was done by Imipenem-EDTA double disc synergy test, Imipenem-EDTA combined disc test, Modified Hodge test and MBL E-test.
Results: Out of 168 A.baumannii isolates, 85 (50.59%) were imipenem resistant. Among these 85 isolates, 57 (67.05%) were MBL positive by DDST, 69 (81.18%) by CDT, 85 (100%) by MHT and all these 85 isolates were confirmed to be MBL positive by MBL E-test method.
Conclusion: Combined disc test, Modified Hodge test & E-test are equally effective to detect MBL production. However, considering the cost constraints of E-test, simple MHT and CDT can be used. They are easy, economical and can be incorporated into routine testing in laboratories to monitor the emergence of MBLs in MDR A.baumannii.
MDR Acinetobacter baumannii (MDR AB), Combined disc test (CDT), Modified Hodge test (MHT), MBL E-test.
Introduction
Acinetobacter baumannii has emerged as one of the most troublesome pathogens for health-care institutions globally. Over the last 16 years it has developed remarkable ability to acquire resistance determinants. Along with its intrinsic and acquired resistance mechanisms, it has the uncanny ability to survive for prolonged period throughout a hospital environment. Its ability to survive under a wide range of environmental condition, makes it a frequent cause of outbreaks of infection and endemic health-care associated pathogen. This multi-drug resistant and Pan resistant A.baumannii is threatening the current antibiotic era.
As reported from reviews dating back to1970s, hospital acquired pneumonia is still the most common infection caused by Acinetobacter. In most recent times, infections involving urinary tract, blood stream, CNS, skin, soft tissues, surgical sites and bones have been reported. Significant advances have been made in understanding this multi-drug resistant organism in last two decades [1].
A.baumannii is often resistant to wide variety of antimicrobials, including carbapenems. Carbapenem group of antibiotics play a vital role in the management of nosocomial infections due to gram negative organisms because of their broad spectrum activity and the ability to hydrolysis by most of the β-lactamases including ESBLs [2]. Carbapenem resistance in A.baumannii is due to variety of combined mechanisms such as hydrolysis by β-lactamases, alterations in the outer membrane proteins and penicillin-binding proteins and increased activity of efflux pumps [3,4]. In addition to this, acquired resistance is mediated by OXA-type carbapenemases and metallo β-lactamases (MBLs). The MBLs efficiently hydrolyse all the β-lactams, except for Aztreonam, in-vitro. Three groups of MBLs (IMP, VIM & SIM) and three groups of CHDLs (OXA group) have been reported in A.baumannii [5,6]. Therefore detection of MBL-producing multi-drug resistant A.baumannii (MDR AB) is crucial for the optimal and modified therapy and also to initiate effective control of the dissemination of resistance.
Few Indian studies have documented the presence of MBLs in A.baumanni. No extensive study has been conducted in Karnataka, especially in and around Mangalore,India regarding the detection of MBL and Carbapenemase producing Acinetobacter sps. Hence the aim of this study was to detect the MBL producing strains among MDR AB, isolated from clinical specimens in this geographical area by various phenotypic tests and their comparative evaluation.
Materials and Methods
This study was conducted in coastal Karnataka, Southern India for a duration of one year. A total of 168 strains of A.baumannii were collected from various clinical samples such as blood, pus, urine, respiratory secretions and other body fluids such as ascitic fluid and synovial fluid, including the patients from ICUs. The multi-drug resistant (MDR) isolates which were resistant to Imipenem were selected and tested for MBL and Carbapenemase production by the following phenotypic tests:
Imipenem-EDTA Double Disc Synergy Test (DDST)
The IMP-EDTA double disc synergy test was performed to detect MBL production [7]. The overnight broth cultures of test isolates along with standard control strains (opacity adjusted to 0.5 McFarland opacity standard) were inoculated onto Mueller-Hinton agar plates as lawn culture according to the CLSI recommendations. After drying, a 10 μg Imipenem disc was placed on the lawn culture with a distance of 15 mm centre to centre from a blank disc. 10 μl of 0.5 M EDTA was added to the blank disc and incubated overnight. Presence of an enlarged zone of inhibition towards EDTA disc was interpreted as positive for MBL production [Table/Fig-1].
Showing Imipenem-EDTA Double Disc Synergy Test (DDST)

Imipenem-EDTA Combined Disc Test (CDT)
The test isolates along with standard control strains (opacity adjusted to 0.5 McFarland opacity standard) were lawn cultured on Mueller-Hinton agar plate as recommended by CLSI. After drying, two 10 μg Imipenem discs were placed on the lawn culture with 20 mm distance from centre to centre of the discs. 10 μl of 0.5 M EDTA was added to one of the imipenem discs and incubated overnight. Isolates showing ≥7 mm increase in the inhibition zone size of Imipenem-EDTA disc than the Imipenem disc alone were considered as MBL producers [8] [Table/Fig-2].
Showing Imipenem-EDTA Combined Disc Test (CDT)
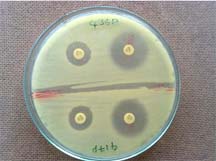
Modified Hodge Test (MHT)
All the isolates were subjected to Modified Hodge test, a screening test which helps in detection of Carbapenemases [3]. Escherichia coli ATCC 25922 (an indicator organism sensitive to carbapenems) was cultured in peptone water to achieve 0.5 McFarland opacity standard and was lawn cultured onto a Mueller-Hinton agar plate using sterile cotton swab. After drying, 10 μg Imipenem disc was placed at the centre of the plate on the lawn culture and an overnight growth of test strain was heavily streaked from the edge of the Imipenem disc outwards, to the periphery of the plate in four different directions. The plates were incubated at 37oC overnight. The presence of a distorted zone (Clover-leaf shaped zone of inhibition) was considered as positive test. The test was repeated with Imipenem + 10 μl 0.5 M EDTA. To determine the effect of Zinc ions on the test and to overcome the equivocal or false-negative results, the test was again repeated with 10 μl of 50 mM ZnSO4 solution (Prepared by dissolving 1438 mg of ZnSO4.7H2O in 100 ml deionized water and sterilized by autoclaving) which is added to Imipenem-EDTA disc [Table/Fig-3].
Showing Modified Hodge Test (MHT)
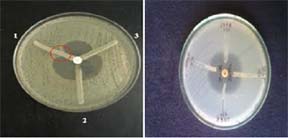
MBL E-Test
All the Imipenem resistant isolates were subjected to E-test [9] to detect Mimnimun inhibitory concentration (MIC) ratio and to confirm MBL production. The E-test MBL strip (bioMerieux SA, France) containing a double sided seven-dilution range of Imipenem (IP) (4 to 256 μg/ml) and Imipenem (1 to 64 μg/ml) in combination with a fixed concentration of EDTA (IPI) was used for MBL detection. The test was done according to manufacturer’s instructions (E-test technical manual, bioMerieux SA, France). MIC ratio of ≥8 for the 2 reagent sides or a phantom zone between IP/IPI or deformation of either ellipse was indicative MBL production [Table/Fig-4].

Results
Among 168 Acinetobacter baumannii isolates, 40 (23.80%) were isolated from pus, 33 (19.64%) from blood, 30 (17.86%) from urine, 63 (37.50%) from respiratory secretions and 2 (1.19%) from other body fluids. 83 (49.40%) isolates among the 168 A. baumannii were Imipenem sensitive and 85 (50.59%) Imipenem resistant. All the 168 isolates were multi-drug resistant and showed 100% sensitivity to Colistin and Tigecycline.
The results of comparative evaluation of MBL production by four Phenotypic tests are shown in [Table/Fig-5]. In Modified Hodge test, addition of 0.5M EDTA to Imipenem disc improved the test performance whereas addition of 50 mM ZnSO4 to Imipenem-EDTA disc did not show any difference in the test performance.
Comparative evaluation of MBL production by various Phenotypic tests (85 Isolates)
| Sl.no | Phenotypic tests | Positive Number (n) | Percentage (%) | Negative Number (n) | Percentage (%) |
|---|
| 1. | Double Disc Synergy Test | 57 | 67.05 | 28 | 32.94 |
| 2. | IMP-EDTA Combined Disc Test | 69 | 81.18 | 16 | 18.82 |
| 3. | Modified Hodge Test | 85 | 100 | 00 | - |
| 4. | MBL E-Test | 85 | 100 | 00 | - |
Discussion
Multi-drug resistant A.baumannii (MDR AB) is a significant pathogen in health care settings where it causes a multitude of infections that include septicaemia, pneumonia, meningitis, urinary tract infection and wound infections. Less frequently it also causes infections of skin and soft tissues, abdominal and CNS infections. The infection tend to occur in immunosuppressed patients, in patients with serious underlying diseases and in those subjected to invasive procedures and treated with broad spectrum antibiotics [1] especially in ICUs. Nosocomially acquired MDR AB pose a real challenge to the clinician.
Carbapenems are generally the last resort in the treatment of life threatening infections caused by MDR AB. However, emergence of Carbapenem hydrolysing β-lactmases of Ambler class B (MBLs) and class D (Oxacillinases/CHDLs), which are proved to be the most important mechanism of carbapenem resistance, have caused serious problem in treatment of MDR AB. These organisms are resistant to carbapenems, fluoroquinolones and aminoglycosides and have been reported from several countries and also from various hospital settings in India [10–14]. Colistin resistant isolates are rare but occurrence is reported [15]. By acquiring various kinds of resistance mechanisms, A.baumannii has evolved as one of the most difficult nosocomial pathogens to control and treat.
Simple and accurate tests are needed to detect MBL producers. There are no standard guidelines available for detection of MBL. Different studies have reported the use of various methods. Most of the studies have used Imipenem-EDTA double disc synergy test, Imipenem-EDTA combined disc test, Modified Hodge test and MBL E-test. According to those studies, MBL production ranged from 7% to 65%. [Table/Fig-6] shows the comparative study.
Comparative Studies by other authors
| Sl.No | Authors and year of publication | Number of Strains tested | DDST | CDT | MHT | E-Test |
|---|
| 1. | Lee k, et al., 2003[7] | 73 | 45 (61.64%) | - | 41 (56.16%) | - |
| 2. | Walsh TR, et al.,2002 [19] | 130 | - | - | - | 83 (63.85%) |
| 3. | Jesudasan M,et al., 2005[17] | 50 | 36 (72%) | - | 28 (56%) | - |
| 4. | Behera B, et al.,2008 [9] | 63 | 36 (57.14%) | 48 (76.19%) | - | 30 (47.62%) |
| 5 | Irfan S et al.,2011 [2] | 100 | - | 83 (96.6%) | - | - |
| 6 | Amudhan SM et al.,2011 [3] | 116 | - | 92 (79.3%) | 113 (97.4%) | - |
| 7 | John S et al.,2011[18] | 242 | 36 (14.8%) | - | 36 (14.8%) | - |
| 8 | Present study | 168 | 57 (67.05%) | 69 (81.18%) | 85(100%) | 85 (100%) |
Several studies have reported the use of DDST as one of the convenient methods for the detection of Ambler class B MBL production and the positivity has varied from 14.8% - 72%. [16–18]. According to their findings, DDST was found to be more reliable and reproducible with high rate of positivity. However, in our study DDST showed the lowest positivity and our result was similar to the study conducted by Behra et al., The disadvantage of DDST was subjective interpretation of result in some instances. In our study, CDT was found to be superior to DDST and was in accordance with other published studies [9].
Modified Hodge test is a simple method for screening MBL producing isolates. It was originally described by the Centre for Disease Control for Carbapenemase detection in Enterobacteriaceae. It has displayed high efficiency for detection of Carbapenemase. However, occasional isolates show false positive results which is may be due to minor carbapenem hydrolysis by CTX-M or AmpC enzymes.[1] Performance of MHT can be more reliable when Imipenem discs with 50mM ZnSO4 and 0.5M EDTA is used [7].
Previously published data has shown low positivity of MHT when compared to other tests, which vary from 14.8% - 56.16%.[7,17,18] However our study has shown high positivity which is in accordance with the results of Amudhan SM et al., Though CLSI does not advocate the use of MHT for detection of Carbapenemase production in non-fermenting gram negative bacilli, several authors have found MHT with Imipenem, EDTA and ZnSO4 as a useful screening test for Carbapenemase production [3].
E-test is a quantitative technique for determining the MIC of antimicrobial agents against microorganisms and for detection of resistance mechanisms. E-test MBL strip based on a combination of a β-lactam substrate and a β-lactam or MBL inhibitor was specifically designed to detect clinically relevant MBLs. The E-test MBL strips (Imipenem-Imipenem+EDTA [Ip-IpI]) has the ability to detect MBLs (both chromosomal and plasmid mediated) in aerobic and anaerobic bacteria. We, in accordance with other published studies,[9,19] found that MBL E-test to be very sensitive for detection of MBLs. Since we screened only Carbapenem resistant isolates with MBL E-test, it might have accounted for increased rate of positivity. Another possibility of higher positivity in our study might be the disseminated multi-drug resistance in Acinetobacter and its counteracting mechanisms occurring in the recent scenario, compared to the earlier times.
Conclusion
Our study showed that MHT and E-test were equally efficient to detect MBL production, followed by Combined disc test. Simultaneous existence of different carbapenemases is a problem to reckon with and should be seriously considered for alternative and newer therapeutic strategies, strict infection control measures and continuous surveillance. Easy detection methods are required for each of these in routine clinical labs. However, considering the cost constraints of E-test, simple CDT and MHT can be used which are easy, economical and can be incorporated into routine testing in labs to monitor the emergence of MBLs and carbapenemases in MDR AB. Initial screening of the putative carbapenemase producers will help to organise intervention and early directed therapy.
[1]. Peleg AY, Seifert H, Paterson DL, Acinetobacter baumannii: Emergence of a Successful PathogenCli Microbiol Re 2008 21(3):538-82. [Google Scholar]
[2]. Irfan S, Zafar A, Guhar D, Ahsan T, Hasan R, Metallo-β-lactamase-producing clinical isolates of Acinetobacter spp and Pseudomonas aeruginosa from intensive care unit patients of a Tertiary care HospitalIndian J Med Microbiol 2008 26(3):243-5. [Google Scholar]
[3]. Amudhan SM, Sekar U, Arunagiri K, Sekar B, OXA Beta-lactamase-mediated carbapenem resistance in Acinetobacter baumanniiIndian J Med Microbiol 2011 29(3):269-74. [Google Scholar]
[4]. Lin MF, Kuo HY, Yesh HW, Yang CM, Sug CH, Tu CC, Emergence and dissemination of blaOXA-23 carrying imipenem-resistant Acinetobacter spp in a regional hospital in TaiwanJ Microbiol Immunol Infect 2011 44:39-44. [Google Scholar]
[5]. Andriamanantena TS, Ratsima E, Rakotonirina HC, Randrianirina F, Ramparany L, Carod JF, Dissemination of multidrug resistant Acinetobacter baumannii in various hospitals of Antananarivo, MadagascarAnn Cli Microbiol & Antimicrob 2010 9:17 [Google Scholar]
[6]. Qi C, Malczynski M, Parker M, Scheetz MH, Characterization of Genetic Diversity of Carbapenem-Resistant Acinetobacter baumannii clinical strains collected from 2004 to 2007J Cli Microbiol 2008 46(3):1106-9. [Google Scholar]
[7]. Lee K, Lim YS, Yong D, Yum JH, Chong Y, Evaluation of the Hodge test and the Imipenem-EDTA Double-Disk Synergy Test for Differentiating Metallo-β-Lactamase Producing Isolates of Pseudomonas spp and Acinetobacter sppJ Cli Microbiol 2003 41(10):4623-9. [Google Scholar]
[8]. Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y, Imipenem-EDTA disc method for differentiation of metallo β-lactamases producing clinical isolates of Pseudomonas spp & Acinetobacter sppJ Cli Microbiol 2002 40:3798-801. [Google Scholar]
[9]. Behra B, Mathur P, Das A, Kapil A, Sharma V, An Evaluation of four different Phenotypic techniques for detection of metallo-β-lactamase producing Pseudomonas aeruginosaIndian J Med Microbiol 2008 26(3):233-7. [Google Scholar]
[10]. Rahbar M, Mehrzan H, Aliakbari NH, Prevalence of antibiotic-resistant Acinetobacter baumannii in a 1000-bed tertiary care hoapital in Tehran, IranIndian J Pathol Microbiol 2010 53:290-3. [Google Scholar]
[11]. Sohrabi N, Akhi MT, Farajnia S, Nahaei MR, Rezaei MA, PER-1-type extended spectrum b-lactamase producing Acinetobacter baumannii isolated from clinical specimens in Tabriz, IranPak J Med Sci 2010 26(4):769-72. [Google Scholar]
[12]. Prashanth K, Badrinath S, Nosocomial infections due to Acinetobacter species: Clinical findings, risk and prognostic factorsIndian J Med Microbiol 2006 24:39-44. [Google Scholar]
[13]. Karthika RV, Rao RS, Sahoo S, Shashikala P, Kanungo R, Jayachandran S, Phenotypic and Genotypic assays for detecting the prevalence of metallo-β-lactamases in clinical isolates of Acinetobacter baumannii from a South Indian tertiary care hospitalJ Med Microbiol 2009 58:430-5. [Google Scholar]
[14]. Shete VB, Ghadage DP, Muley VA, Bhore Av, Multidrug resistant Acinetobacter Ventilator-associated PneumoniaLung India 2010 27(4):217-20. [Google Scholar]
[15]. Lee K, Yong D, Jeong SH, Chong Y, Multi-drug Resistant Acinetobacter spp: Increasingly Problematic Nosocomial PathogensYonsei Med J 2011 52(6):879-91. [Google Scholar]
[16]. Arakawa Y, Shibata N, Shibayama K, Kurokawa H, Yagi T, Fujiwara H, Convenient Test for Screening Metallo-β-Lactamase-Producing Gram Negative Bacteria by using Thiol CompoundsJ Cli Microbiol 2000 38(1):40-3. [Google Scholar]
[17]. Jesudasan MV, Kandathil AJ, Balaji V, Comparison of two methods to detect Carbapenemase and Metallo-β.0-lactamase production in clinical isolatesIndian J med Res 2005 121:780-3. [Google Scholar]
[18]. John S, Balagurunathan R, Metallo beta-lactamase producing Pseudomonas aeruginosa and Acinetobacter baumanniiIndian J Med Microbiol 2011 29(3):302-4. [Google Scholar]
[19]. Walsh TR, Bolmstrom A, Qwarnstrom A, Gales A, Evaluation of a new E-test for detecting Metallo-β-lactamases in Routine Clinical TestingJ Cli Micribiol 2002 40(8):2755-9. [Google Scholar]