Use of free soft tissue autograft procedures has expanded the available armamentarium in periodontics, which is an attempt which has been made, which fulfills the goal of reconstruction of normal functional architecture.
Palatal masticatory mucosa usually serves the purpose of harvesting of full epithelized grafts or relatively thick connective tissue grafts for use in periodontal plastic surgeries done (Miller) at oral and occasionally non-oral sites, such as eyelid displacements or retractions [1] lower lid spacers [2] or lip re-constructions [3]. In periodontics, free grafts of the palatal mucosa are used for root coverage, for increasing width of the attached gingiva, for alveolar ridge augmentation and for vestibuloplasty, to increase the supportive area of the denture base [4,5]. Since graft connective tissue may act as a “Natural GTR (Guided Tissue Regeneration) membrane”, allowing new attachment of previously denuded roots, it is used as subperiosteal and intraosseous connective tissue graft for treatment of pocket reductions [6].
Palatal tissue thickness shows intra-individual and inter-individual variations in height, length [7] and thickness [8–10]. A graft of adequate dimension is required for obtaining ideal results. Thickness of the graft may include palatal epithelium, connective tissue (lamina propria) and thin layer of sub mucosa. The optimal graft thickness is 2 mm for one step or for a direct approach [11] and it is 1-1.5 mm for an indirect or a two step procedure [12]. Thickness of palatal masticatory mucosa is evaluated by using both invasive and non invasive methods. Palatal mucosa has major advantages, such as being histologically identical to keratinized attached mucosa of alveolar ridge; it also provides a comfortable tissue base for the denture, with regards to resilience and quantity of epithelium [5]. It also supplies adequate donor tissues for both isolated and multiple adjacent areas of recession. The other advantages are, its predictability; colour match of the connective tissue grafts with the adjacent tissue and minimal adverse palatal post operative squealae if there are any. The main disadvantages of this graft are, limited amount of tissue being available to make the graft and also, potential risk of damaging the greater palatine artery (GPA) due to variations in the anatomy of the palatal vault [7]. A free gingival grafting can also cause unpredictability and infrequent side effects like exostoses [13,14].
There may be other confounding factors that influence the palatal submucosal thickness, such as racial and genetic factors, and body weight. It is not unreasonable to hypothesize that body weight makes an effect on the amount of adipose tissue present in the palatal submucosal layer, that results in an increased thickness of the palatal mucosa. The effect of the body mass index on palatal mucosal thickness was investigated and it has shown positive results [9].
Till date, only few reports are available on the masticatory mucosal thickness in dentate individuals. The thickness, volume and course of GPA are important, for obtaining sufficient volume without injuring the vessels, in order to predict the prognosis.
Materials and Methods
This study was conducted in the Outpatients Department of Periodontics, Rajah Muthiah Dental College and Hospital, Annamalai University, for a period of 06 months. Thirtysix subjects were included under two groups (of ages 14-29 years and 30-59 years), with 18 sujects in each group. All the subjects were aware of the procedure and informed consents were taken from them. Patients were grouped as:
Group I: Younger age group of between (14-29 years)
Group II: Older age group of between (30-59 years)
Individuals with a history of any palatal or tuberosity surgery, a history/presence of a stomatological disease in the palate or a tuberosity, pregnancy or lactation, use of any medication which had possibly affected the periodontal tissues, presence of any fixed partial denture between the upper canine and the second molar, those who wore any removable device in the upper arch, smokers (current and former smokers), those with tooth malpositions, rotations, or spacing were included.
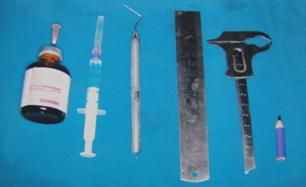
The materials used in this study were a UNC – 15 probe, local anaesthesia, a 2 ml syringe and a needle (Dispovan, India), Boley gauge, self cure acrylic resin (DPI, India), Haematoxylin pencil, and a dental stone [Table/Fig-1].
Methodology
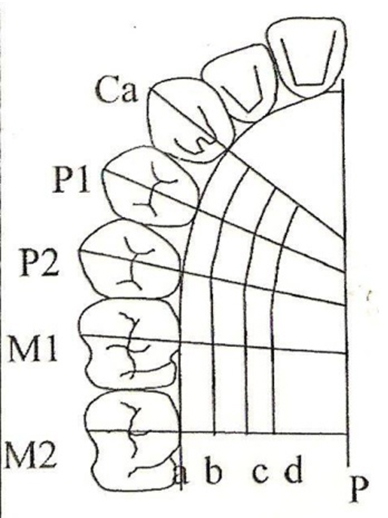
In the first visit, upper arch impressions were made and study models were prepared for each individual. Clear acrylic stents were fabricated for each subject. Fifteen measurement points were marked on the study models. Line p was the midline of palate. Line a was gingival margin. Lines b, c and d ran parallel to Line a. Line b ran 3 mm away from the gingival margin. Lines c and d were located at ¼th and ½th the distance between line b and line p respectively. Lines ca, P1 and P2 were positioned at the mid- palatal aspect of the canine (ca), first premolar (p1), and second premolar (p2), respectively. Lines (1st molar M1) and (2nd molar M2) ran through the mesio-lingual cusp of the first and second molars, respectively [Table/Fig-2].
Fifteen measurement points were marked on the study models. Line p is the midline of palate. Line a - gingival margin. Line b, c and d run parallel to line a. Line b run 3mm from the gingival margin. Line c and d are located ¼ and ½th the distance between line b and line p respectively. Line ca, P1 and P2 are positioned at the mid- palatal aspect of the canine (ca), first premolar (p1), and second premolar (p2), respectively. Lines (1st molar M1) and (2nd molar M2) run through the mesio-lingual cusp of the first and second molars, respectively
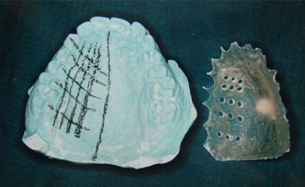
A fissured diamond bur was used to create holes at the marked measurement points on the stent, at 900, to provide a consistent location for the assessment of mucosal thickness [Table/Fig-3].
Measuring points marked on the stent at 900 to provide a consistent location for the assessment of mucosal thickness
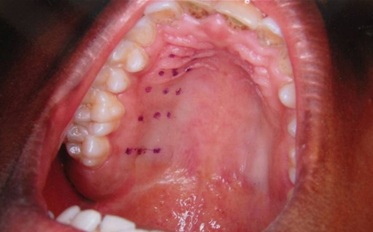
In the second visit, the palate was anaesthetised with spray and greater palatine nerve and incisive nerve were blocked with 0.1 ml and 0.05 ml of 2% lidocaine, 1:80,000 epinephrine. Fifteen measurement points were recorded, 20 minutes after the injection with the stent was placed on the upper arch, by using an indelible haematoxylin pencil, according to the holes which were made on the stent [Table/Fig-4,5].
Fifteen measurement points were marked with haematoxylin pencil using stent

Fifteen measurement points are seen after removal of the stent
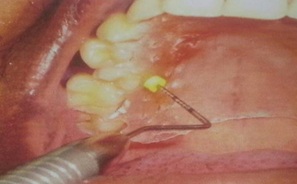
Without the stent, the thickness of palatal masticatory mucosa was measured by “Bone Sounding” by using a graded periodontal probe (UNC-15 periodontal probe) and a rubber stopper which was in contact with the mucosal surface. The probe with the rubber stopper securely in place, was then lined to a 0.5 mm scale, a sterile, stainless steel ruler, and the value was consistently rounded up to the nearest 0.5 mm. When the measurement point was in the rugae area. The base of the rugae and not the hill was chosen as the measurement point. All measurements were made twice by the same investigator, with an interval of 10 minutes between them. The average of the 2 measurements was considered as the final measurement [Table/Fig-6].
Measuring thickness palatal masticatory mucosa with Unc-15 Probe
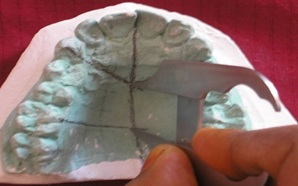
To measure the maximum available length and height of the palatal donor graft tissue, the supposed course of the GPA was marked with a pencil on the maxillary dental casts. All measurements were made with a Boley gauge to the nearest millimetre [Table/Fig-7,8].
Measuring the Length of Palate on the study model using Boley gauge
Measuring the Height of Palatal Vault Boley gauge
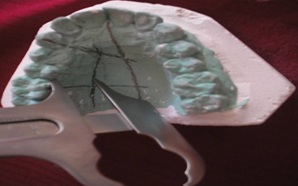
The emergence of the GAP was located midway between the gingival margin of the second molar and the midline raphe. The GPA was assumed to be located at the junction of the vertical and horizontal walls of the palatal vault. The length of the palatal vault donor site was measured from the mid-palatal aspect of the canine, to the mid-palatal aspect of the second molar, at the greater palatine level. The height of the palatal vault donor site was measured from the gingival margin to the level of the greater palatine, at the interproximal aspect and at the aspect of each tooth, from the canine to the second molar.
The mean heights of palatal vault donor site (i.e., distance from the gingival margin to the GPA) were corresponded with the following teeth numbers and zones.
3 – Canine zone
3-4 – Proximal zone between canine and first premolar
4 – First premolar zone
4-5 – Proximal zone between first premolar and second premolar
5 – Second premolar
5-6 – Proximal zone between second premolar and first molar
6 – First molar
6-7 – Proximal zone between first and second molars
7 – Second molar zone
UNC – 15 is a 15 mm colour coded probe, with colour coding at 5, 10, and 15 mm markings. The diameter of this probe is 0.45 mm, which is almost similar to the outer diameter of the 25 gauge needle.
Boley gauge is a vernier calliper like gauge which is graduated in millimetres (mms), which is used to measure thicknesses of various dental materials. Its accuracy is one tenth of a mm.
Results
In the present study, results demonstrated the participants had thinnest mucosa at the canine region, which was increased in the more distal parts, and became thickest at the second molar area. The mean thickness ranged between 1.7 mm at the canine, on line b to 5.58 mm at the second molar on line d [Table/Fig-9]. Overall, mean thickness of palatal masticatory mucosa ranged between 2.47 to 2.65 mm [Table/Fig-10].
Mean thickness of palatal masticatory mucosa in (mm) at three points in individual tooth by age group
| Tooth | Line | Group I (Age 14-21 years) Mean | Group II (Age 30-59 years) Mean | p-value |
|---|
| Canine | B | 1.728 | 1.972 | <0.001 |
| C | 2.122 | 2.311 | 0.012 |
| D | 2.244 | 2.628 | <0.001 |
| Premolar I | B | 1.817 | 1.917 | 0.029 |
| C | 2.322 | 2.578 | 0.007 |
| D | 2.661 | 2.833 | 0.025 |
| Premolar II | B | 1.606 | 1.856 | 0.006 |
| C | 2.356 | 2.550 | 0.155 |
| D | 2.639 | 3.144 | <0.001 |
| Molar I | B | 1.522 | 1.650 | 0.139 |
| C | 2.272 | 2.278 | 0.877 |
| D | 2.933 | 2.956 | 0.725 |
| Molar II | B | 1.350 | 1.867 | <0.001 |
| C | 2.844 | 3.378 | <0.001 |
| D | 5.550 | 5.589 | 0.757 |
Overall mean thickness of palatal masticatory mucosa in (mm) by age group and gender
| Age | Male | Female | t-value | p-value |
|---|
| Mean (SD) in mm | Mean (SD) in mm | | |
| Group I (14-21) | 2.47 (0.126) | 2.38 (0.09) | 1.589 | 0.132 |
| Group II (30-59) | 2.65 (0.038) | 2.524 (0.076) | 4.49 | <0.001 |
Thus, older age group showed a thicker mucosa as compared to younger group and from distal of canine and premolar areas.
In females; Group I had a significantly thinner mucosa with a mean thickness of 2.38 mm than males who showed a mean thickness of 2.47 mm [Table/Fig-10]. Females had a thinner mucosa in Group II, with a mean thickness of 2.52 mm than males who showed a mean thickness of 2.65 mm [Table/Fig-10], which were in concordance with the findings of the study which was conducted by Vandana and Savitha [15]. Thus, males showed a thicker mucosa as compared to females [Table/Fig-11].
Mean thickness of palatal masticatory mucosa in (mm) all individuals and by age group and gender
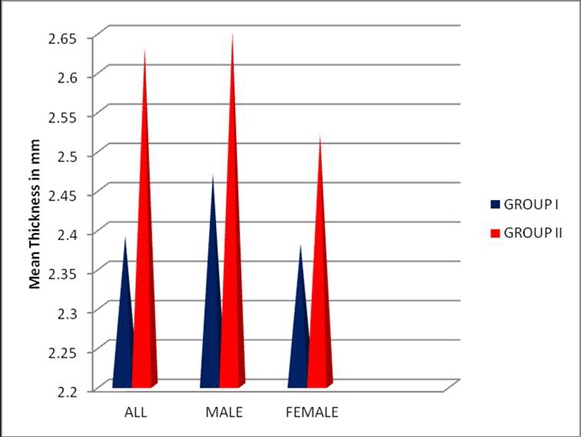
Mean thickness of palatal masticatory mucosa seen in all individuals ranged from 2.39 – 2.63 [Table/Fig-12].
Mean thickness of palatal masticatory mucosa (mm) in all individuals
| All individuals | Mean (SD) | t-value | p-value |
|---|
| Group I (14-21) | 2.39 (0.99) | 4.726 | <0.001 |
| Group II (30-59) | 2.63 (0.96) |
In the present study, mean height of the palatal vault at the donor site ranged from 12.06 (1.0) mm at the mid palatal aspect of canine to 14.0 (1.20) mm at the interproximal aspect between the second premolar and first molar, which may be due in part to ethnic differences [Table/Fig-13]. The second premolar and the second molar areas showed the greatest height. The mean height which was measured from second premolar area to the second molar area was significantly greater in men than in women.
Mean height (mm) of palatal vault donor site by age, gender and both at individual sites
| Group | Mean | Location | T3 | T3-4 | T4 | T4-5 | T5 | T5-6 | T6 | T6-7 | T7 |
|---|
| Group I | M | Mean (SD) | 12.06 (0.36) | 12.50 (0.34) | 12.82 (0.28) | 13.53 (0.40) | 14.07 (0.63) | 14.66 (0.633) | 15.10 (0.568) | 15.50 (0.667) | 15.75 (0.667) |
| F | Mean (SD) | 11.72 (0.750) | 12.47 (0.377) | 12.68 (0.280) | 13.15 (0.469) | 13.32 (0.381) | 13.72 (0.403) | 13.85 (0.351) | 14.20 (0.346) | 14.18 (0.259) |
| Group Ii | M | Mean (SD) | 12.08 (0.371) | 12.73 (0.452) | 13.02 (0.352) | 13.68 (0.399) | 14.11 (0.404) | 14.79 (0.433) | 15.08 (0.230) | 15.35 (0.242) | 15.80 (0.258) |
| F | Mean (SD) | 11.925 (0.149) | 12.375 (0.231) | 12.475 (0.276) | 12.938 (0.320) | 13.137 (0.37) | 13.72 (0.483) | 14.063 (0.410) | 14.388 (0.242) | 14.45 (0.185) |
| Two-way ANOVA results | f-value | Age Sex Age/Sex | 0.53 2.631 0.355 | 0.281 2.404 1.813 | 0.004 11.19 4.150 | 0.054 17.371.812 | 0.220 29.7 0.523 | 0.143 33.93 0.143 | 0.487 67.520.710 | 0.017 63.041.403 | 1.278 111.00.591 |
| p-value | Age Sex Age/Sex | 0.472 0.115 0.555 | 0.599 0.131 0.188 | 0.951 0.002 0.050 | 0.818 <0.001 0.188 | 0.642 <0.001 0.475 | 0.707 <0.001 0.707 | 0.490 <0.001 0.406 | 0.896 <0.001 0.245 | 0.269 <0.001 0.448 |
The mean length of the harvesting area for Group I was 28.3 (3.5) mm and that for Group II was 34.8 (2.7) mm, which ranged from 24 to 46 mm. The mean length was 30.5 (5) mm for women and it was 32.4 (3.9) mm for men [Table/Fig-14].
Mean length of the palatal vault by age and gender (in mm)
| Mean (SD) | p-value |
|---|
| Group I | 28.3 (3.5) | 0.73 |
| Group II | 34.8 (2.7) |
| Male | 32.4 (3.9) | 0.23 |
| Female | 30.5 (5) |
Discussion
Reconstruction of damaged periodontal tissues has taken an increasingly important role in periodontal plastic surgeries. Sub epithelial connective tissue graft due to its high predictability provides a better aesthetic outcome with an adequate thickness of the tissue. One of the contraindications of palatal graft harvesting relates to palatal area that fails to provide adequate donor tissue, either as a result of palatal anatomic form or insufficient thickness of the soft tissue which is harvested. The surgical approach may need to be altered accordingly, depending on the availability of adequate tissue. The options can be, harvesting from the ideal palatal donor site or augmenting the palatal connective tissue area, which can subsequently be used for connective tissue grafting [15].
Since dimensions of the donor graft tissues play an important role in the clinical outcomes of periodontal plastic surgical procedures, it is important to determine the available dimensions of the palatal donor tissues, prior to attempting surgical harvesting.
The greatest length (anterior-posterior dimension) can be found in a large palate [16].The thickest tissue can be found in the area between the mesial line angle of the palatal root of the first molar and the distal line angle of the canine [2,8,9,17,18]
The greater and lesser palatine nerves and blood vessels gain entrance into the palate by passing through the greater and lesser palatine foramina. The foramina locations vary, but generally, neurovascular bundle may be located 7 to 17 mm from the CEJ (Cemento enamel junction) of the maxilla.
Wara-aswapathi et al., [18] assessed the overall thickness of palatal mucosa, which ranged between 2.0-3.7 mm among participants, which was in concordance with the findings of the present study.
Although the palatal mucosa of the younger age group was thinner than that of the older group, the mucosal thickness of the group ranged from 1.7 to 5.5 mm, which suggested that a sufficient volume of palatal donor tissue could be obtained for doing sub epithelial connective tissue grafting in this group.
Thus, the results showed a thicker mucosa in older age group as compared to that seen in younger group and distance between distal of canine and premolar areas appeared to be most appropriate donor site for crrying out grafting procedures.
It is possible that the thickness of the ortho keratinized epithelial layer of the hard palate mucosa increases with age, resulting in the palatal mucosa which is seen in older subjects. It might also be due to gingival tissue which is found to become more coarse and dense with age. In addition, unlike gingiva, hard palate possesses a sub mucosal layer which contains various amounts of adipose tissue and small mucous glands [19]. The structural change which occurs in the epithelium and connective tissue with age is controversial [20,21].
The present study showed a thicker mucosa in males as compared to that in females, which was in concordance with findings of the study conducted by Vandana and Savitha [22].
The palatal neurovascular bundle, which is housed in a palatal groove and is located approximately 7 to 17 mm from the CEJ of the upper premolars and molars (depending on the shape of the palatal vault), may have an effect on the measurement, if the probe penetrates into the neurovascular structures. However, in the present study, there were no clinical indications which showed that the neurovascular bundle had been penetrated (such as bleeding after probing, haematoma formation or paraesthaesia), when the mucosal thickness at the canine and premolar areas were compared to that which was reported by Studer et al., [8].
Thin (<0.05mm) or intermediate (0.05-0.75mm) thickness grafts are used for increasing the width of attached gingiva and thick (0.75-1.25mm) or full thickness (>1.25mm) grafts are used for ridge augmentations and root coverage procedures [18].
The present study demonstrated that the canine and premolar areas were the most appropriate sites to harvest a palatal mucosa of thickness 1.8 mm ± 0.8 mm, which were in concordance with thicknesses of 1.5-2.0 mm [23] 2.0 mm [11]. In younger and older subjects, from all the areas on lines b, c, d, mesial to second molar, it was possible to harvest epithelialized grafts of thickness 1.5 mm, except from the first molar on lines b and c. The palatal root of first molar acts as an “anatomical barrier”, thus limiting donor tissue volume. It is also possible to harvest connective tissue grafts by excluding the epithelium (310μm).
The optimal thicknesses of palatal mucosa, demonstrated by different studies were: 0.8-1.3 mm,[24] 0.5-0.36 mm,[25] 0.9 mm, [26] 1.0-1.5 mm, 0.9 [27]. Free palatal grafts of 0.9mm thickness proved to be functionally sufficient, regardless of whether they healed on denuded alveolar bone or on a periosteal bed [26].
In the present study, mean height of the palatal vault donor site ranged from 12.06±1.0 mm at the mid palatal aspect of canine to 14.07±1.20 mm at the interproximal aspect, between the second premolar and first molar, which may be due in part to ethnic differences. The second premolar and the second molar areas showed the greatest height. The mean height which was measured from second premolar area to the second molar area was significantly greater in men than in women. Hence, it is possible to harvest a connective tissue graft which measures 5mm in height, from the premolar area, in all cases.
Monnet-Corti et al., [7] measured the height of the palatal vault donor site, which ranged from 12.07 ± 2.9 mm at the mid-palatal aspect of canine to 16.2 ± 2.2 mm at the interproximal aspect, between the second premolar and first molar. The second premolar and the second molar areas showed the greatest height. The mean height which was measured from second premolar area to the second molar area was significantly greater in men than in women. They found that it was possible to harvest a connective tissue graft which measured 5mm in height, from the premolar area, in all cases.
The mean length of the harvesting area for Group I was 28.3 ± 3.5 and for Group II, it was 34.8 ± 2.7 mm, which ranged from 24 to 46 mm. The mean length was 30.5 ± 5 mm for women and it was 32.4 ± 3.9 mm for men.
Limitations
Thickness of palatal masticatory mucosa may be influenced by other factors such as genetics and body mass. In the present study the thickness was assessed by using a probe. Further studies are necessary which make use of devices like ultrasound for obtaining a more appropriate thickness of the masticatory mucosa without causing inconvenience to the individuals.
Interpretation and Conclusion
Within the limits of the present study, the mean thickness of palatal masticatory mucosa in younger age group appeared to be thinner when compared with the older individuals and the thickness of palatal masticatory mucosa was not significant with the genders. Thus, the ideal donor tissue site is located between the canine to mid palatal aspect of first molar without hampering the neurovascular bundles.