Esthetics is a very important reason for which patients seek Orthodontic treatment. The introduction of fixed appliances has several advantages such as shorter treatment times, precise and more controlled tooth movements. However, their advent has brought enamel white spot lesions to the attention of Orthodontists [1]. The banding and bonding of Orthodontic appliances to teeth increases the number of plaque retention sites and as a result maintenance of oral hygiene becomes more difficult. The low pH of plaque adjacent to Orthodontic brackets hinders the remineralization process and decalcification of enamel can occur.
Initial enamel demineralization usually manifests itself clinically as a “white spot lesion” (WSL) [2]. The levels of acidogenic bacteria, such as Streptococcus mutans, become significantly elevated in Orthodontic patients.
The characteristic appearance of these lesions is caused by an optical phenomenon owing to subsurface tissue loss and is exaggerated by thorough drying [2]. It lasts approximately two years with smooth surface lesions increasing up to 50% in prevalence during treatment [3].
Many methods like improving oral hygiene, low carbohydrate intake, use of fluoride containing sealants and adhesives can decrease or prevent white spot lesions. Various compliance free methods have been attempted [4]. Newer methods such as the use of Ozone, TiF4 have also been proposed [5,6]. Following the realization that fluoride was responsible for reduced recurrent decay surrounding silicate restorations, there have been numerous attempts to incorporate Fluorides into dental restorative materials and cements [7].
This study focuses on comparison of three different Fluoride releasing products: MI PASTE PLUS with RECALDENT (Casein phosphopeptide-amorphous calcium phosphate (GC Corporation, GC Asia Dental Pvt.Ltd, Singapore), FLUOR PROTECTOR (Cervitec, Ivoclar vivadent, United States) and PHOSFLUR (Colgate, New york) in preventing demineralization around brackets without compromising their shear bond strength (SBS) or tensile bond strength.
Materials and Methods
MI PASTE PLUS with RECALDENT
Fluor Protector varnish
Phosflur mouth rinse
Therapeutically extracted Premolar teeth
Acrylic blocks with teeth embedded
Mounting jig made of acrylic for applying force
0.022′ Preadjusted Edgewise MBT prescription stainless steel premolar brackets (GEMINI, 3M UNITEK)
Distilled water for cleaning the teeth after extraction
Saline to store teeth after cleaning the extracted teeth
Bonding accessories
Etchant (Scotch bond 3M ESPE, United States)
Light cure adhesive primer (3M UNITEK, Monrovia, California)
Transbond XT Adhesive (3M UNITEK, Monrovia, California)
Applicator brush
Three way syringe
Bracket holder
Bracket positioning gauge
Mouth mirror
Explorer
Pumice and Polishing Rubber cup
Light Emitting Diode curing unit
For evaluation of adhesive remnant
Simple Microscope (10 x)
For evaluation of acid etched enamel surfaces [Table/Fig-1]
Scanning Electron Microscope (JSM 6510).
Scanning Electron Microscope

Methodology
Sample for the Study
A total of 200 therapeutically extracted human premolars were obtained from patients reporting to the Department of Orthodontics and Dentofacial Orthopedics for Orthodontic treatment. Teeth were stored in physiologic saline (0.1% NaCl) and study was conducted within a span of 15 days. Acrylic blocks ranging from 3 x 2.5 cm dimensions were prepared and teeth were embedded up to CEJ with their buccal surfaces made perpendicular to the base of the block.
Distribution of the Sample
The experimental and control teeth were randomly divided into 4 groups:
Sample Preparation
1. GROUP I ( CONTROL)
BONDING PROCEDURE: The buccal surface of the teeth were washed with distilled water and dried using oil and moisture free air from a three way syringe for 5 seconds. The enamel was then treated with 37% Orthophosphoric acid for 30 seconds, washed away with a spray of water for 10 seconds. The tooth surface was then air dried till a white chalky appearance was seen on the surface. The primer was applied with the help of an applicator brush. The adhesive was then applied to the base of the metal bracket.
The bracket was then positioned on the tooth surface along the long axis of the tooth at a predetermined position from the occlusal surface with the help of a bracket positioning gauge. The adhesive was cured using a LED. The adhesive was cured from the mesial and distal aspects for 10 seconds each.
2. GROUP II (CPP-ACP)
The buccal surface of teeth was applied with a pea sized amount of CPP-ACP paste. It was kept for 30 minutes as per the manufacturer’s instructions before the bonding procedure. Bonding procedure is same as that of control group.
3. GROUP III ( FLUOR PROTECTOR)
The buccal enamel surface was thoroughly cleaned and dried. A thin layer of Fluor Protector varnish was applied using the viva brush. It was evenly dispersed with an air syringe and after 45 minutes bonding was done.
4. GROUP IV (PHOSFLUR)
The buccal surface of teeth was immersed in 10 ml of Phosflur mouth rinse for one minute and bonding procedure was performed after 30 minutes as per manufacturer’s instructions.
Evaluation of Shear Bond Strength
Shear Bond Strength was tested using a Universal Testing Machine (AUCE/TEQIP/MET/E-5). A jig was prepared by attaching a sharp chisel-shaped rod to a block of acrylic of dimensions 7 x 3.5 cm. It was attached to upper jaw of the machine. The acrylic block containing the embedded teeth was secured in the lower jaw of the machine such that the bracket base of the teeth parallel the direction of the shear force at a crosshead speed of 1 mm/minute until bracket failure. The force required to dislodge the bracket was recorded.
Assessment of Adhesive Remnants
After debonding, all samples were examined under 10X magnification to assess adhesive remnants on tooth surface using the ADHESIVE REMNANT INDEX (ARI) system. The scoring criteria for evaluation was:
1 = All the adhesive remained on the tooth.
2 = More than 90% of the adhesive remained on the tooth.
3 = More than 10% but less than 90% of the adhesive remained on the tooth.
4 = Less than 10% of the adhesive remained on the tooth.
5 = No adhesive remained on the tooth.
Evaluation of Depth of Etching
SEM observations were carried out to observe the acid etched enamel surfaces pretreated with or without the agents using a Scanning Electron Microscope (JSM 6510). The results obtained were subjected to statistical evaluation.
Results
As per [Table/Fig-2,3] it can be seen that, the mean strength value is highest for PHOSFLUR followed by FLUORPROTECTOR , CPPACP and lowest for CONTROL.
Mean, SD of shear bond strength (in MPA) for all the four groups (Control, Cppacp, Fluorprotector, Phosplur)
| Group | n | Means | SD. |
|---|
| Group I | 50 | 7.0462 | 0.8838 |
| Group II | 50 | 10.5368 | 1.1307 |
| Group III | 50 | 13.4854 | 1.8243 |
| Group IV | 50 | 15.3658 | 2.4546 |
Comparison of four groups (Control, CPPACP, Fluorprotector, Phosflur) with respect to shear bond strength (MPa) values
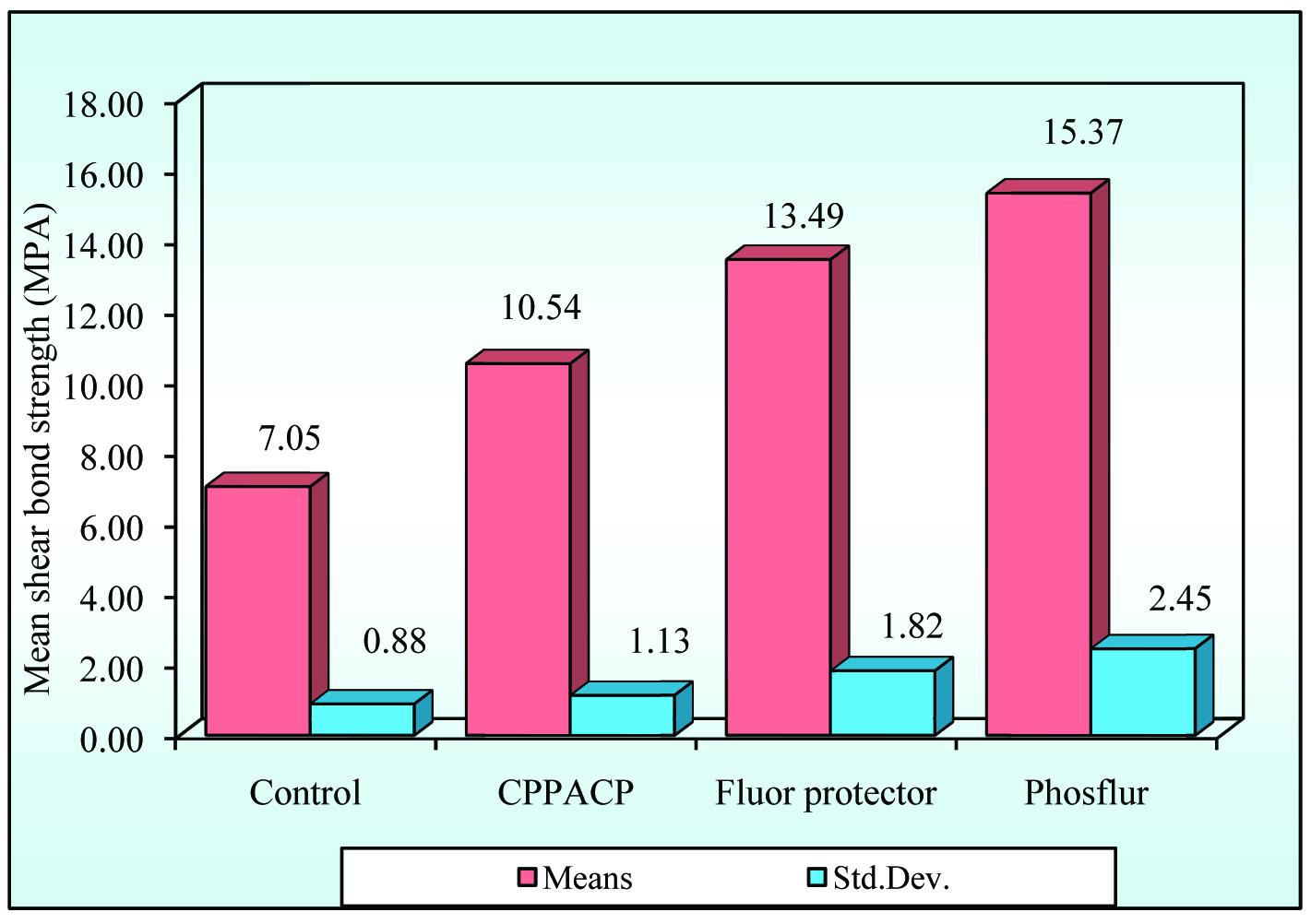
From the [Table/Fig-4], it can be seen that, there is a significant difference in strength values between the four groups at 5% level of significance and Group IV samples have significantly higher strength (MPa) other groups.
Comparison of four groups (Control, CPPACP, Fluorprotector, Phosplur) with respect to Shear bond strength (MPa) values by one way ANOVA
| Source of variation | Degrees of freedom | Sum of squares | Mean sum of squares | F-value | P-value |
|---|
| Between groups | 3 | 1980.16 | 660.05 | 231.3362 | 0.0000* |
| Within groups | 196 | 559.23 | 2.85 | | |
| Total | 199 | 2539.39 | | | |
*p<0.01
From this it can be seen that, significant difference in strength was observed between Group I and Group IV, Group I and Group III, Group I and Group IV (p<0.05) at 5% level of significance and group IV has higher mean strength value (MPa) when compared to Group I, Group II and Group III.
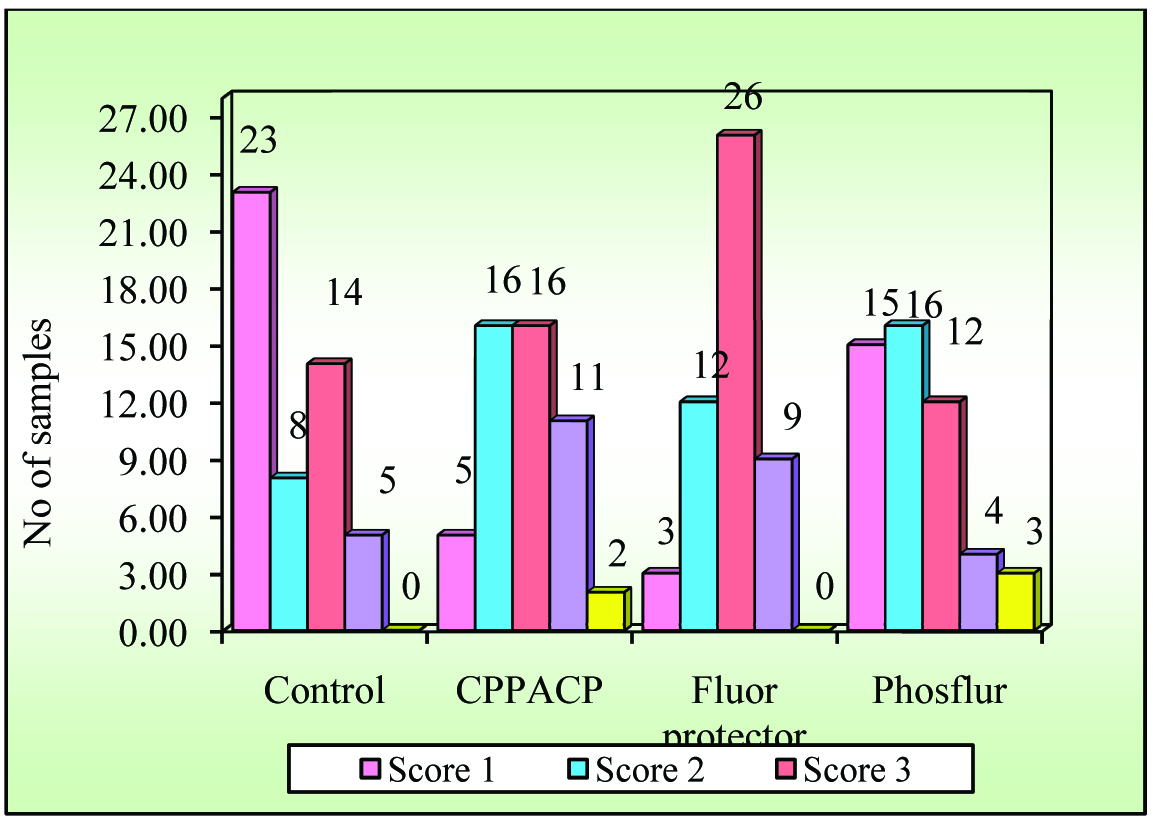
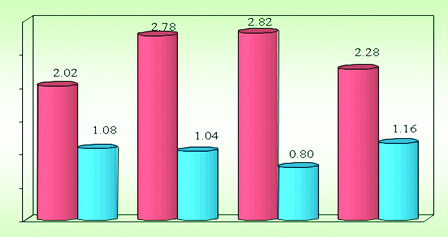
According to the Mean and SD of ARI scores as represented in [Table/Fig-5,6and7], the mean ARI score is highest for Fluorprotector (2.82 ± 0.80) followed by CPPACP (2.78 ± 1.04), PHOSFLUR (353.49±23.47) and lowest for Control (2.02 ± 1.08).
Mean, SD of ARI scores according to four groups
| Group | N | Means | Std.Dev. |
|---|
| Control | 50 | 2.02 | 1.08 |
| CPPACP | 50 | 2.78 | 1.04 |
| Fluor protector | 50 | 2.82 | 0.80 |
| Phosflur | 50 | 2.28 | 1.16 |
Comparison of four groups (Control, CPPACP, Fluorprotector, Phosplur) with respect to ARI score values
Comparison of four groups (Control, CPPACP, Fluorprotector, Phosplur) with respect to ARI score values
As per [Table/Fig-8], all the groups showed a higher percentage of ARI scores of one.
Comparison of four groups with respect to ARI scores by Chi square test
| Groups | Score 1 | % | Score 2 | % | Score 3 | % | Score 4 | % | Score 5 | % | Total |
|---|
| Control | 23 | 46.00 | 8 | 16.00 | 14 | 28.00 | 5 | 10.00 | 0 | 0.00 | 50 |
| CPPACP | 5 | 10.00 | 16 | 32.00 | 16 | 32.00 | 11 | 22.00 | 2 | 4.00 | 50 |
| Fluor protector | 3 | 6.00 | 12 | 24.00 | 26 | 52.00 | 9 | 18.00 | 0 | 0.00 | 50 |
| Phosflur | 15 | 30.00 | 16 | 32.00 | 12 | 24.00 | 4 | 8.00 | 3 | 6.00 | 50 |
| Total | 46 | 23.00 | 52 | 26.00 | 68 | 34.00 | 29 | 14.50 | 5 | 2.50 | 200 |
Chi-square = 42.6471
df = 12
p = 0.00003*
*p<0.01
From the [Table/Fig-9], it can be seen that, there is a significant difference in ARI score values between the four groups and the group III samples have significantly higher ARI score value than those of the other groups.
Comparison of four groups with respect to ARI scores by Kruskal Wallis ANOVA test
| Groups | Means | Std.Dev. | Median | Sum of ranks | h-value | p-value |
|---|
| Group I | 2.02 | 1.08 | 2.00 | 3880.50 | 20.7897 | 0.0001* |
| Group II | 2.78 | 1.04 | 3.00 | 5784.50 | | |
| Group III | 2.82 | 0.80 | 3.00 | 6014.50 | | |
| Group IV | 2.28 | 1.16 | 2.00 | 4420.50 | | |
*p<0.01
From the [Table/Fig-10], it can be seen that significant difference in ARI scores was observed between the groups and Group III has higher mean ARI scores when compared to Group I, Group II and Group IV.
Pair wise comparison of four groups with respect to ARI scores by Mann-Whitney U-test
| Groups | Mean | SD | Median | Sum of ranks | u-value | z-value | p-value |
|---|
| Group I | 2.02 | 1.08 | 2.00 | 2055.00 | | | |
| Group II | 2.78 | 1.04 | 3.00 | 2995.00 | 780.000 | -3.2401 | 0.0012* |
| Group I | 2.02 | 1.08 | 2.00 | 2001.00 | | | |
| Group III | 2.82 | 0.80 | 3.00 | 3049.00 | 726.000 | -3.6124 | 0.0003* |
| Group I | 2.02 | 1.08 | 2.00 | 2374.50 | | | |
| Group IV | 2.28 | 1.16 | 2.00 | 2675.50 | 1099.500 | -1.0375 | 0.2995 |
| Group II | 2.78 | 1.04 | 3.00 | 2475.00 | | | |
| Group III | 2.82 | 0.80 | 3.00 | 2575.00 | 1200.000 | -0.3447 | 0.7303 |
| Group II | 2.78 | 1.04 | 3.00 | 2864.50 | | | |
| Group IV | 2.28 | 1.16 | 2.00 | 2185.50 | 910.500 | -2.3405 | 0.0193** |
| Group III | 2.82 | 0.80 | 3.00 | 2940.50 | | | |
| Group IV | 2.28 | 1.16 | 2.00 | 2109.50 | 834.500 | -2.8644 | 0.0042* |
*p<0.01, **p<0.05.
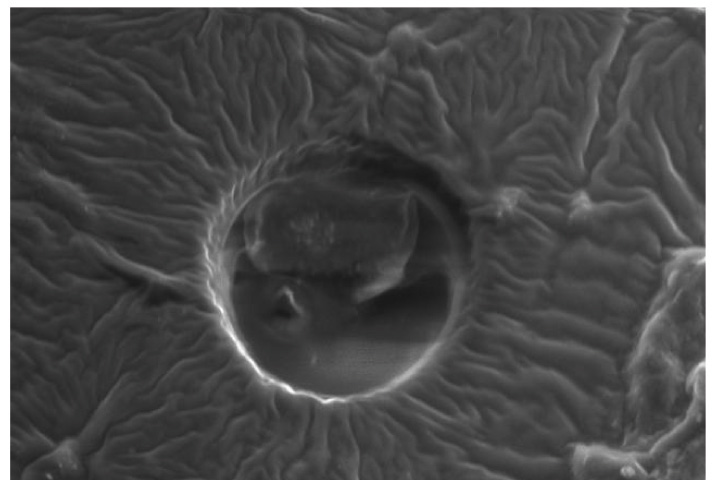
Representative SEM images of the etched enamel specimens pretreated with and without CPPACP, FLUOR PROTECTOR, PHOSFLUR are shown in [Table/Fig-11,12,13and14].
SEM observation of acid-etched enamel surface
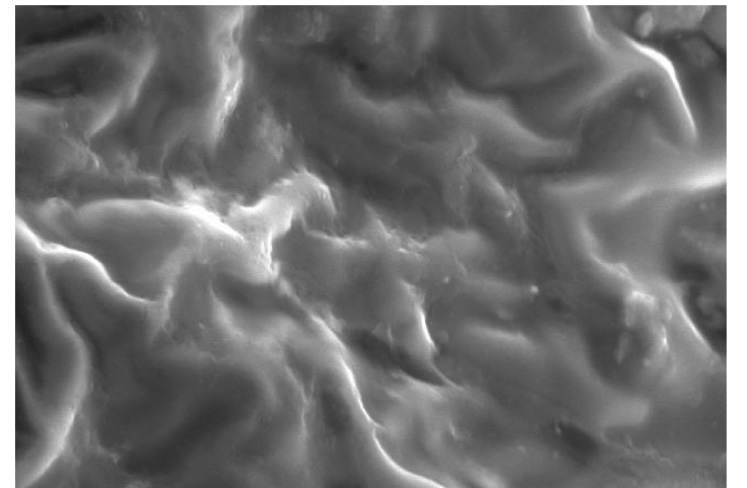
SEM observation of acid-etched enamel surface after pretreatment with CPP-ACP
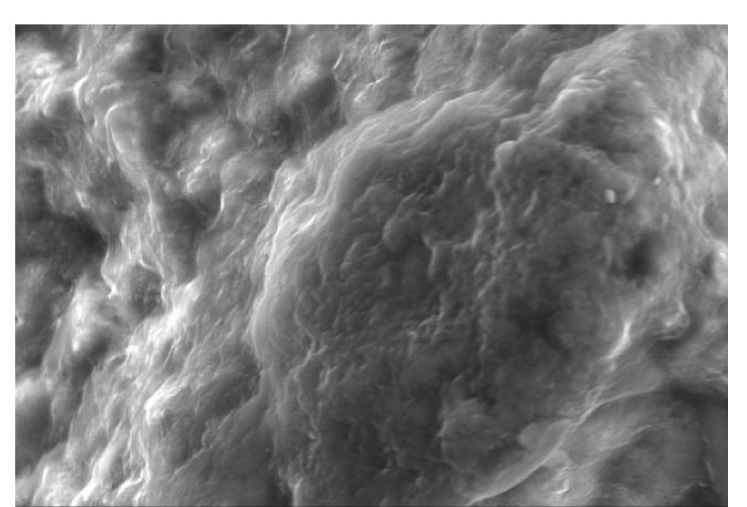
SEM observation of acid-etched enamel surface after pretreatment with FLUOR PROTECTOR
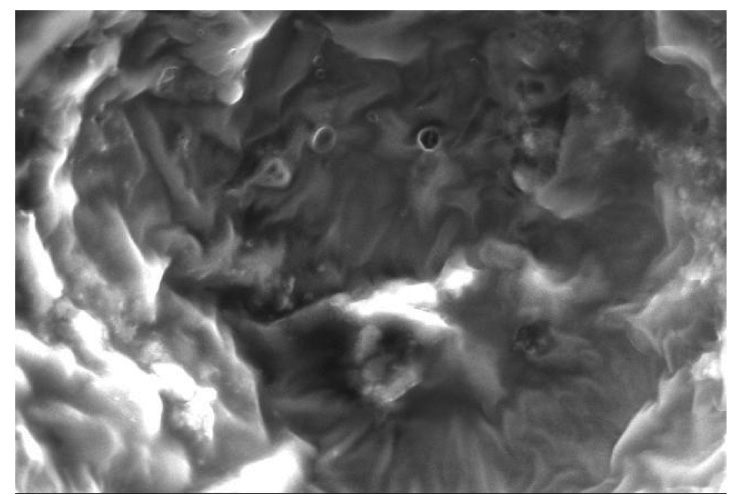
SEM observation of acid-etched enamel surface after pretreatment with PHOSFLUR
Discussion
The technique of bonding Orthodontic brackets to enamel with acrylic resin dates back to 1965 [8]. The procedure included acid-etch technique to better adhere the brackets to enamel [9].
Orthodontic appliances as such are not a cause of demineralization or caries, but creation of new retentive sites could result in oral hygiene problems when Orthodontic appliances are worn [10].
In a previous study, it was reported that 49.6% of Orthodontic patients experienced some degree of white spot lesion formation which is a result of demineralization of enamel in and around the bracket area [11]. Caries and enamel decalcification can be greatly reduced by maintaining good oral hygiene, applying topical Fluorides, and/or using a Fluoride-containing dentifrice during Orthodontic treatment [12]. In other studies, however, the topical application of Fluoride to enamel surface before etching with phosphoric acid did not negatively affect the bond strength [13]. Topical Fluoride application works primarily through (1) a reduction in the rate of dissolution in the demineralization phase in acidic conditions, (2) the enhancement of remineralization at the crystal surface, and (3) the inhibition of bacterial enzymes [14].
In an earlier study conducted by Dunn it was suggested that Orthodontic brackets bonded to teeth with an ACP containing composite material failed at significantly lower forces than brackets bonded to teeth with conventional resin-based composite Orthodontic cements. So the question that arises is whether the disadvantage of low bond strength due to the effect of the material outweighs its advantage as a protector against demineralization.
Recent studies however show that CPP-ACP application can cause increased shear bond strength of brackets when light-cured adhesive is used. In this in vitro study the effects of pretreatment of CPP-ACP on Shear bond strength (SBS) of Orthodontic brackets was examined.
CPP-ACP was found to have higher bond strength compared to the bond strength recommended by Reynolds and Whitlock et al. as adequate for Orthodontic purposes. SBS was favorably affected when the enamel was pretreated with CPP-ACP.
Fluor protector varnish strengthens enamel by protecting it against demineralization and promotion of remineralization by forming Calcium Fluoride layer (CaF2). Several investigators have shown that the etching effect of phosphoric acid on enamel surfaces pretreated with topical Fluoride agents was impeded, causing reduced bond strengths of dental resins. In our study, Shear bond strength (SBS) was favorably affected when the enamel was pretreated with Fluor Protector.
Phosflur mouth rinse has APF formulation that promotes remineralization and strengthens teeth. In our study, Shear bond strength (SBS) was favorably affected when the enamel was pretreated with Phosflur.
This in-vitro study clearly indicates that significant differences in Shear bond strength (SBS) existed between Group I (Control) and Group II (CPP-ACP) , Group III (Fluor Protector) and Group IV (Phosflur). Group IV (Phosflur) having APF formulation showed the maximum Shear bond strength in comparison to control and the other groups.
Assessment of residual debris following bond failure was evaluated with the Adhesive Remnant Index (ARI) index. It has been stated that the most common failure site when stainless steel brackets are used is the adhesive/bracket base interface and consequently the bond strength at the etched enamel and adhesive interface is greater than that at the bracket base/adhesive interface. Failure at the base and adhesive interface results in adhesive remnants being firmly attached to the enamel. In our study too, the most common failure site was the adhesive/bracket interface for all the four groups.
In context to the shear bond strength values, Group I (Control) having the least bond strength and Group IV (Phosflur) having highest bond strength, showed similar residual debris at the debonding surfaces. When comparing Group IV (Phosflur) with Group II (CPP-ACP) and Group III (Fluor Protector) it was found that the amount of residual debris at the debonding surfaces was proportional to the increase in shear bond strength values. However, with the work by Maijer and Smith, it is confirmed that debonding of Orthodontic adhesives was easy after crystal growth conditioning. It is supported by the fact that Fluoride is known to enhance crystal growth.
Thus, it can be explained that it is not a case of differing bond strengths at the separate interfaces that governs failure site; it is probably caused by stress concentration and consequent crack formation that progresses to bond failure.
When the adhesive material is used in very thin sections, as in the bonding system, the site of failure becomes influenced by the design of the bracket base and by the type of adhesive material used.
Scanning Electron Microscope (SEM) observations were carried out on the enamel surfaces pretreated with CPP-ACP, Fluorprotector, Phosflur. Images as in [Table-Fig-5–8] revealed relatively rougher etched enamel surfaces than Control. A rougher enamel surface results in a greater adhesive area and more resin tags available for bonding.
In our study, Group I (Control) and Group IV ( Phosflur) showed a Type 1 etching pattern, Group II (CPP-ACP) showed a Type 2 etching pattern and Group III (Fluor Protector) showed Type 3 etching pattern.
From the observations of this study, we can presume that CPP-ACP, Fluor Protector and Phosflur favourably affect the Shear bond strength of Orthodontic brackets.
Conclusion
Based on findings and within the limitations of the present in-vitro study, the following conclusions can be drawn:
SBS values of CPPACP, Fluor Protector, and Phosflur are higher than Control.
ARI scores indicate the role of Fluoride in enhancing crystal growth.
There is increase in surface area of etching after pretreatment with the fluoride rich materials.
*p<0.01
Chi-square = 42.6471
df = 12
p = 0.00003*
*p<0.01
*p<0.01
*p<0.01, **p<0.05.