Esophageal squamous cell carcinoma (ESCC), which is the predominant histologic subtype of esophageal cancer, is considered as one of the fatal cancers worldwide. It occurs at a high frequency rate in Asia and South America [1]. Kashmir valley and north-east India have reported highest incidence of this cancer in India [2,3]. Despite significant advances in the field of surgery and therapeutic strategies, the prognosis of these patients remains dismally poor. The overall survival rate at five years is being estimated as low as 14% [4].
Esophageal squamous cell carcinogenesis is a multifactorial process with influence of local environmental conditions, lifestyle and genetic predisposition [5]. Betel quid chewing, a common habit in south-east Asia has been found to increase the risk of developing ESCC by 4.7-13.3 fold [6]. This assumes importance since using fermented areca nuts with any form of tobacco is a common habit in north-east India and might be a potential risk factor of ESCC in this region. Many molecular alterations occur in esophageal carcinogenesis. Investigation into these protein alterations may provide clues to discover novel biomarkers for improving diagnosis and guiding targeted therapy [7]. A number of biomarkers have been studied, out of which the inactivating mutations in tumour suppressor genes such as p53 and retinoblastoma (Rb) genes have proved to be of particular importance for the development of esophageal cancer. These gene products play ominous role in cell cycle control. Mutations of the p53 gene as well as overexpression of p53 protein have been associated with ESCC. Moreover loss of Rb gene and reduced expression of retinoblastoma protein (pRb) have been frequently reported in ESCC. So, any abnormality in p53-pRb pathway might lead to esophageal squamous cell carcinogenesis and its progression [8].
Although combined analysis of p53 protein and pRb has been reported from other high incidence regions, similar clinical analysis has not been attempted in a high incidence region of north east India, particularly Meghalaya, which is different from the mainland India in terms of ethnicity, lifestyle, food habits and cultures.
Materials and Methods
The study was conducted on 30 cases of ESCC from January 2010 to June 2011 presented in outpatient department or being admitted at NEIGRIHMS. The diagnosis was based on clinical symptoms and signs, endoscopic examination and histopathological examination of the esophageal endoscopic biopsies and/or resection biopsies. Total 10 cases had a total or subtotal esophagectomy. None had chemotherapy or radiotherapy.
Staging was done in all the patients with radiological investigations like radiological contrast (barium swallow), computed tomography scans, endoscopic ultrasonography and magnetic resonance imaging. After the initial radiological staging, patients in operable conditions were operated for esophageal resection. The rest of the patients were classified as inoperable based on their clinicopathological conditions. Staging was determined as per the Union International Cancer TNM classification guidelines [9].
Morphologic Evaluation
Samples were fixed in 10% neutral buffered formalin and embedded in paraffin. Staining was done with hematoxylin and eosin Histologically confirmed ESCC tumors were graded as well differentiated (G1), moderately differentiated (G2) or poorly differentiated (G3).
Immunohistochemical Analysis of P53 and Prb
Assessments of p53 and pRb were done immunohistochemically by Horse Radish Peroxidase (HRP) method. The paraffin embedded sections of the tumour were stained by a monoclonal antibody raised against p53 (Biogenex, CA, USA), and pRb (Novocastra Laboratories Ltd, Newcastle, UK).
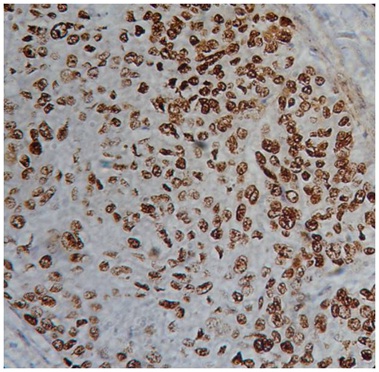
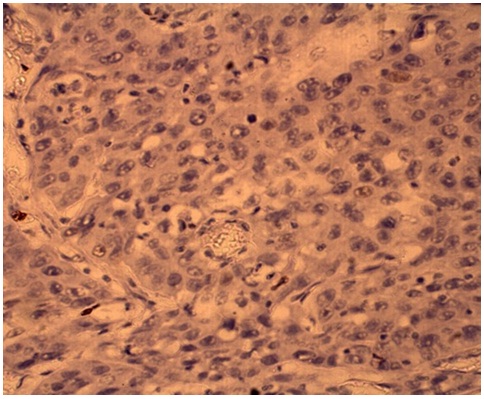
p53 and pRb expression was determined by two independent observers, who were blinded to the clinical details of the patients. Immunoreactivity was classified into the following three categories based on the percentage of tumour cells showing nuclear reactivity: less than 10%, 10% to 50% and more than 50%. Immunointensity was classified as no immunostaining (-), weakly immunostaining (+), weak immunostaining (++) and strongly positive immunostaining (+++).Tumours showing p53 expression in more than 50% of cells with strongly positive immunostaining were considered as positive [8] [Table/Fig-1]. pRb positive cancer cells were assessed as follows: if more than 50% of tumour cells stained for pRb, tumours were considered pRb positive [8] [Table/Fig-2].
ESCC showing strong nuclear p53 positivity (x400)
Negative pRb immunoreactivity in ESCC (x400)
Control Group
Normal esophageal epithelia, which represented a positive control, were included in each run and the negative control section was run without the primary antibody. Thirty healthy subjects were randomly selected, who were referred for upper gastrointestinal health examination and diagnosed as normal, based on physical examination and were histologically proven not to have a cancerous lesion. They were genetically unrelated to the cases and they had no previous cancer history. The control group was matched to the case group by age (± 5 years) and gender. The benign controls showed p53 expression in less than 20% of cells with weak intensity mainly confined to the basal epithelial cells. In pRb immunostaining, adequate nuclear staining was observed in the benign controls in all cases.
Statistical Analysis
The differences of patients’ distribution between positive and negative expression of p53 and pRb in individual variables were evaluated by Chi-Square (χ2) test and Fischer’s exact test. A p-value less than 0.05 was considered statistically significant.
Results
Out of 30 study subjects, there were 20 male and 10 female patients with a male: female ratio of 2:1. The age of the patients ranged from 36 years to 79 years with mean age of 53.2 years. Two cases were well differentiated ESCC (G1), 14 were moderately differentiated ESCC (G2) and rest of 14 were poorly differentiated ESCC (G3). Seven cases were in stage I, 8 in stage II, 11 in stage III and 4 in stage IV. Twenty-five (83.3%) were positive for p53 and 17 (56.7%) were positive for pRb. p53 and pRb expressions were compared in terms of various clinicopathological characteristics [Table/Fig-3 and Table/Fig-4]. All 30 cases were classified into four groups based on p53 and pRb status of tumors (p53-/ pRb+, n=3; p53-/ pRb- , n=2; p53+/ pRb+, n=14; p53+/ pRb-, n=11). Clinicopathological characteristics were analyzed among the four groups [Table/Fig-5].
Distribution of variables in 30 cases of ESCC by p53 status
| Total number (n= 30) | p53 negative (n= 5) | p53 positive (n= 25) | p-value |
|---|
| Tumor size (cm) | 0.042 |
| ≤ 3 cm | 15 | 5 | 10 | |
| > 3 cm | 15 | 0 | 15 | |
| Tumor site | 0.065 |
| Upper 1/3rd (C15.3) | 0 | 0 | 0 | |
| Middle 1/3rd (C15.4) | 12 | 2 | 10 | |
| Lower 1/3rd (C15.5) | 15 | 1 | 14 | |
| Overlapping lesion (C15.8) | 3 | 2 | 1 | |
| Tumor invasion to adventitia | 0.009 |
| No | 13 | 5 | 8 | |
| Yes | 17 | 0 | 17 | |
| Lymph node metastasis | 0.014 |
| Absent | 14 | 5 | 9 | |
| Present | 16 | 0 | 16 | |
| Histologic type of tumor | 0.253 |
| Well differentiated(G1) | 2 | 0 | 2 | |
| Moderately differentiated(G2) | 14 | 1 | 13 | |
| Poorly differentiated(G3) | 14 | 4 | 10 | |
Distribution of variables in 30 cases of ESCC by pRb status
| Total number (n= 30) | pRb negative (n= 13) | pRb positive (n= 17) | p-value |
|---|
| Tumor size (cm) | 1.000 |
| ≤ 3 cm | 15 | 6 | 9 | |
| > 3 cm | 15 | 7 | 8 | |
| Tumor site | 0.693 |
| Upper 1/3rd (C15.3) | 0 | 0 | 0 | |
| Middle 1/3rd (C15.4) | 12 | 6 | 6 | |
| Lower 1/3rd (C15.5) | 15 | 6 | 9 | |
| Overlapping lesion (C15.8) | 3 | 1 | 2 | |
| Tumor invasion to adventitia | 0.015 |
| No | 13 | 2 | 11 | |
| Yes | 17 | 11 | 6 | |
| Histologic type of tumor | 0.980 |
| Well differentiated(G1) | 2 | 1 | 1 | |
| Moderately differentiated(G2) | 14 | 6 | 8 | |
| Poorly differentiated(G3) | 14 | 6 | 8 | |
| Lymph node metastasis | 0.003 |
| Absent | 14 | 2 | 12 | |
| Present | 16 | 11 | 5 | |
Combined analysis of p53 and pRb protein expression in 30 cases of ESCC
| p53-/ pRb+ (n=3) | p53-/ pRb- (n=2) | p53+/ pRb+ (n=14) | p53+/ pRb- (n=11) | p-value |
|---|
| Tumor nvasion to adventitia | 0.001 |
| No | 3 | 2 | 8 | 0 | |
| Yes | 0 | 0 | 6 | 11 | |
| Lymph node metastasis | 0.0007 |
| Absent | 3 | 2 | 9 | 0 | |
| Present | 0 | 0 | 5 | 11 | |
| Histologic type of tumor | 0.753 |
| G1 | 0 | 0 | 1 | 1 | |
| G2 | 1 | 0 | 7 | 6 | |
| G3 | 2 | 2 | 6 | 4 | |
| TNM Stage | 0.042 |
| I | 2 | 2 | 3 | 0 | |
| II | 0 | 0 | 5 | 3 | |
| III | 0 | 0 | 4 | 7 | |
| IV | 1 | 0 | 2 | 1 | |
Discussion
p53 plays an critical role in maintaining the cellular integrity by suppressing the oncogenic transformation, which it does by interrupting the G1 phase of cellular cycle and thus gives time for repairing any DNA damage or apoptosis induction if the damage is irreparable [1]. Alterations of the p53 gene, such as gene mutation, can lead to carcinogenesis, which occurs as a result of loss of regulation of cell growth, DNA repair or apoptosis induction. These alterations can lead to p53 protein stability leading to nuclear accumulations and which can be detected by immunohistochemistry methods [10]. Like p53, pRb also plays a crucial role in regulating the cell cycle by phosphorylation of Rb. Phosphorylation of Rb occurs in an undulating fashion suggesting its role in regulation of cell cycle. Thus inactivation of pRb may lead to uncontrolled cell growth. pRb is inactivated either by mutation of pRb or mutation of genes that regulate pRb. This leads to loss of Rb function and reduced expression of pRb [11,12].
In a study done in Kashmir valley, Murtaza I et al., found 77.7% of p53 positivity in ESCC [13]. Similarly in Iran, Taghavi N et al., found 56.2% p53 positivity and in Japan, Ikeguchi M et al found 41% of p53 positivity in ESCC [8,14]. Also, Ikeguchi M et al., found 43% of ESCC negative for pRb whereas Mathew R et al., demonstrated 75% pRb negativity in ESCC [8,15]. In the present study, the percentage p53 positivity is higher than in other high incidence regions like Kashmir Valley, Iran and Japan [8,13,14]. Also the pRb negativity showed marked variation. This disparity in protein expression may be related to ethnogeographic variation and marked differences in lifestyle, food habits and cultures peculiar to north-eastern region of India.
In the current study, p53 expression showed significant association with tumor size, invasion to adventitia and lymph node metastasis whereas pRb expression was significantly associated with tumor invasion to the adventitia and lymph node metastasis. These results suggest that these protein products, encoded by deregulated p53 and Rb genes, have relation to clinical aggressiveness in the form of tumor invasiveness and metastasizing ability of cancer cells consolidating their role as independent prognostic predictor in ESCC.
A combined analysis of p53 and pRb status was undertaken in this study. The p53+/ pRb- phenotype ESCC was found to have significant correlation with tumor invasion to the adventitia, nodal metastasis and TNM staging. Thus cases with alterations in both p53 and Rb pathways have an aggressive tumor phenotype providing evidence of a co-operative synergistic effect of p53 and Rb pathways on tumor behaviour.
Conclusion
Alterations of p53-pRb pathway play an important role in ESCC and the tumors with p53+/ pRb- phenotype are the most aggressive. The respective deregulated proteins act individually as well as synergistically imparting an aggressive behaviour to the tumor cells and may serve as putative prognostic markers in ESCC.