Conventional method for Post-operative pain relief is administration of opioids (intravenous or intramuscular) which will lead to unwanted complications such as loss of consciousness, respiratory depression, nausea and vomiting, constipation, itching, and prolonged hospital stay [8,9].
Pain control using NSAIDs and anticonvulsants has also been investigated in some studies, although their overdose may have such complications as GI bleeding or sedation [10–12]. Invasive ways of pain control also requires appropriate equipment and clinical conditions that suit the patient. Pain control with Glucocorticoids has already been investigated [13–15], and Glucocorticoids have been used as adjuncts to other methods of treating pain. Glucocorticoids create some useful results such as reduced pain and facilitated recovery after surgery [16–19]. Also studies have shown that Glucocorticoids can reduce fatigue, nausea and vomiting after surgery [20–24]. Methylprednisolone is classified as Glucocorticoids which reduces inflammatory mediators during and after surgery [22,25,26]. Advantages of using Glucocorticoids and their effectiveness after surgery need further clinical studies. Few works have investigated effectiveness of high dosages of Glucocorticoids on reduced pain after major surgeries especially in orthopedic surgeries [27]. The current study continues previous studies by investigating the effect of single-dose methylprednisolone 125 mg on reduced pain in patients with stable intertrochanteric unilateral fracture who were candidate for Open Reduction Internal Fixation (ORIF) surgery electively.
Materials and Methods
The study was a Double Blind Randomized Clinical Trial. The population of the research included 40-75 years people with stable intertrochanteric unilateral fracture who underwent Open Reduction Internal Fixation surgery electively.
The sample size of the main study has been derived from the initial outcome (rest pain score 24 hours) of a pilot study that was conducted with a sample size of n=10. The findings of this pilot study showed pain score was 3 with 2.5 SD by Visual Analog Scale (VAS). A reduction of 50% in VAS in the MP group compared with the placebo group was obtained [14]. Thus, given Alpha error 0.05 and power 90% for the study, sample size was estimated as 66 which are equal to 82 patients including 10% of drop out.
The study adopted convenience sampling approach. Enrolled patient were taken from all the referred patients to the Rasoul-Akram hospital, a referral university general hospital in Tehran, Iran. The selection procedure followed all of the inclusion and exclusion criteria (discussed below) and patient’s informed consent was obtained beforehand. The duration of the study was 12 months: Aug 2012 – Aug 2013.
Inclusion criteria
– 40-75 years people with stable intertrochanteric unilateral fracture.
– Patients with both verbal and written communication abilities who could consciously and independently provide their consent for participation in the study.
Exclusion criteria
– Contraindication for spinal anesthesia.
– Spinal anesthesia failure.
– Intertrochanteric fractures with high energy (unstable).
– Renal failure.
– Severe heart disease.
– Active peptic ulcer.
– Diabetes mellitus type 1 and 2.
– Rheumatoid arthritis.
– Neurologic diseases potentially.
– using steroids or immunosuppressive therapy at the past 6 months.
– Sensitivity to Marcaine / methylprednisolone / fentanyl / NSAID
– Addiction to alcohol, drugs, or cigarettes.
– Mental disorders which potentially affect pain perception.
Patients were randomly assigned to two groups of the size 41 in each group based on a list generated by a computer-derived randomization algorithm. The groups were called methylprednisolone (MP) group and the saline control group (C). In order to minimize differences in surgery process, Open Reduction Internal Fixation technique with Dynamic Hip Screw (DHS) was applied in all surgeries. In order to make the study double blind, the patient, the person who administered medicine, and the person who collected data were unaware of the nature of the medicine and only the operating room technician, who had a recording responsibility, was aware of the prescribed medicine for each patient.
All patients were treated with oxazepam and ranitidine for premedication in the night before surgery and the morning of surgery. After patient’s entry to the operation room, half an hour before surgery, Methylprednisolone (Iran Hormone Co., Iran) as 500 mg vial was distilled with diluted water. Each milliliter diluted vial contained 50 mg MP; 2.5 mL of the diluted vial (125 mg) was injected. In the MP group and in C group 2.5 mL saline was infused. Both infused via covered syringes over 10 minutes. Patients were under standard monitoring including ECG, pulse oximetry and blood pressure. Before spinal anesthesia, patients were hydrated using 5mL/kg saline. Also, 1-2 mg midazolam was injected for sedation. Spinal anesthesia was performed using a 25-gauge spinal needle (Dr. Japan Co., China) with a dose of 15 μg Fentanyl and 2.5 mL 0.5% bupivacaine in lateral position of the L5 - L4 space. Evaluation of the level of anesthesia to T10 level was done using alcoholic cotton.
During the surgery, additional sedation with propofol 1% (15μ/kg/min) was administered intravenously as required. In case systolic blood pressure reduction went over 30 percent or less than 80 mm Hg, 10 mg ephedrine was injected and in case the heart rate dropped below 50 beats per minute, atropine 0.5 mg was injected. Calculation of blood transfusions and fluid therapy was done using the standard procedure; and if the amount of blood loss was greater than 500 mL, the patient was excluded from the study. In order to control the pain after surgery, patients in both groups were treated by continuous intravenous infusion (Autofuser, Acemedical Co., South Korean) containing Apotel (1gr, Uniform Co.) 2.5 gr daily with 4 mL/hr flow. In case the pain was not controlled, if pain score was higher than 3, Pethidine 15 mg was administered to the patient and recorded. In the event of Post-operative nausea and vomiting (PONV) in the recovery, methoclopramid 10 mg was used.
Finally, pain was evaluated in the rest state and 45° flexion of the hip in times 4, 6, 8, 12, 24, 36, and 48 hours after surgery, and while walking with walker and partial wait for a distance between the bed and the room door in times 24, 36, and 48 hours after surgery using VAS tool (0 = no pain, 10 = worse possible pain). Nausea and vomiting in recovery room was evaluated through regular assessment of PONV scores (no nausea & vomiting=0, Nausea only=1, Vomiting Once=2, Vomiting more than once=3), and fatigue was evaluated with the 10-score (NRS 1, fit; 10, fatigued) and recorded [14,28]. In case of PONV≥1, metoclopramide was injected. CRP level in patients before and 4 hours after surgery was measured and recorded. Consumption of opioid (pethidine) and doses were registered. Sedation score was recorded with Pasero Opioid-induced Sedation Scale (POSS) in recovery room [29]. Patients were hospitalized for 4 days and were released following standard release criteria [30].
Data collected were entered into the SPSS 11 statistical software. Frequency and percentage of frequency functions were used for qualitative variables, and means and standard deviations were calculated for quantitative variables. Qualitative data analysis was done using Chi-square test or Fisher Exact test. Quantitative data analysis was done using T-test or Mann-Whitney U-test. For the comparison of pain score and fatigue in the groups, repeated measure ANOVA was used. For the statistical analysis, p-value <0.05 was considered as significant. This study was in compliance with the ethical principles of Helsinki. The study was approved by the ethics committee of Iran University of Medical Sciences and was recorded at IRCT Center (the code number 2013051113182N1).
Results
One hundred and one patients were assessed for eligibility in the trial. Eighty-two were included and were divided into two groups with 41 members in each group [Table/Fig-1].
Flow diagram of patients in the trial. n= number of patient
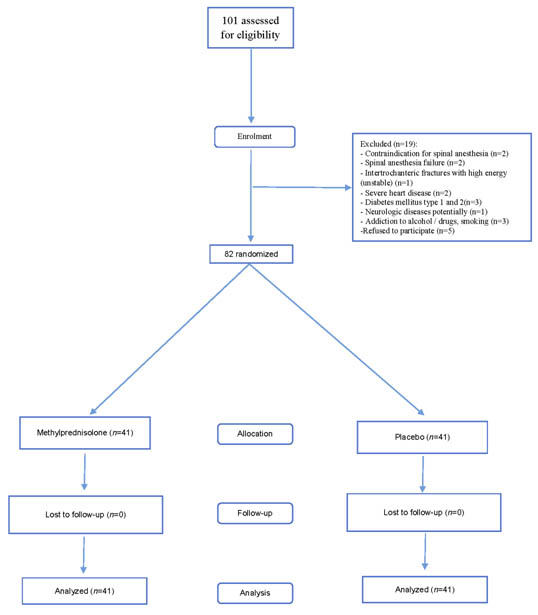
Thirty eight patients (46.3%) were female and 44 patients (53.7%) were male. The mean age of patients was 68.1 ± 7.7 years.
Demographic data of the patients and surgery time are provided in [Table/Fig-2]. No significant statistical difference was observed among the two groups, which denotes appropriate randomization of patients in the two groups.
Demographic and operative data presented as mean ± SD
| Variable | Placebo (n = 41) | Methylprednisolone (n = 41) | p-value |
|---|
| Age (year) | 68.2 ± 7.4 | 67.8 ± 8.1 | 0.84 |
| Gender (female/male) | 21/20 | 17/24 | 0.37 |
| Weight(kg) | 67.2 ± 10.8 | 65.6 ± 14.5 | 0.57 |
| Surgery duratin (minute) | 201.2 ± 33.7 | 213.6 ± 29.4 | 0.08 |
Sedation score was measured in the recovery room and was 2 and 1 in the MP and C groups respectively (p=0.23).
Overall, mean pain score 48 hours after surgery was significantly lower in the MP group in three states at rest, hip flexion, walking [Table/Fig-3].
Pain at rest, upon 450 flexion hip, during walking
| Mean VAS(0-10) | p-value |
|---|
| Placebo (n = 41) | Methylprednisolone (n = 41) |
|---|
| At rest | 2.9 ± 0.8 | 2 ± 0.7 | <0.001* |
| Upon 450 flexion hip | 3.5 ± 0.8 | 2.4 ± 0.7 | <0.001* |
| During walking | 2.9 ± 0.9 | 2.3 ± 1.4 | 0.014* |
| Data presented as mean ± SD., VAS = Visual Analog Scale (0-10). |
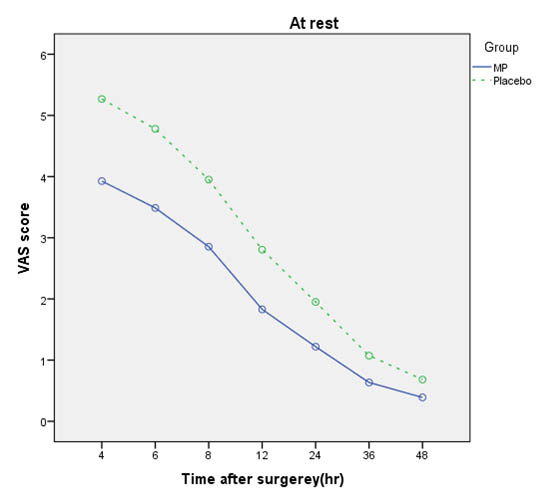
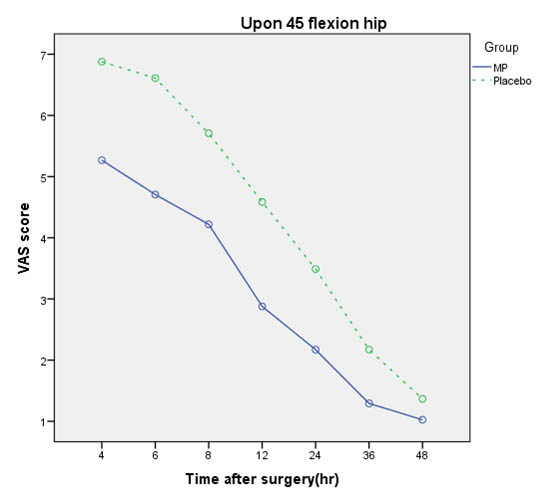
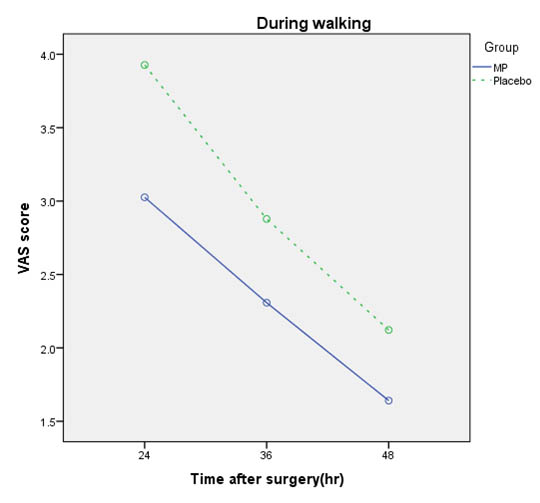
Comparison of pain scores of patients in all states in times 4, 6, 8, 12, 24, 36, and 48 hours after surgery in both groups are shown in [Table/Fig-4,5and6].
Comparison of VAS mean score at rest in Methylprednisolone (MP) and Placebo groups.
Comparison of VAS mean score upon 450 flexion hip in Methylprednisolone (MP) and Placebo groups,
Comparison of VAS mean score during walking in Methylprednisolone (MP) and Placebo groups
Incidence of nausea, fatigue, opioid consumption, and CRP levels were compared for the two groups and are shown in [Table/Fig-7].
Incidence of nausea, fatigue, opioid consumption, CRP levels
Data presented as Mean ± SD, NRS = numerical rating scale (1-10)
| Variable | Placebo (n = 41) | Methylprednisolone (n = 41) | p-value |
|---|
| Number of patient with nausea | 21 (51.2%) | 17 (41.5%) | 0.37 |
| Number of patients with vomiting | 5 (12.2%) | 3 (7.3%) | 0.45 |
| Number of patients requiring opioid consumption | 16 (39) | 13 (31.7%) | 0.49 |
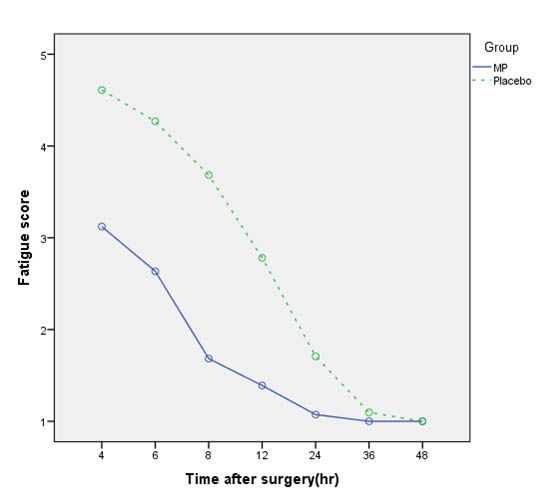
| Fatigue score(NRS) | 4.7 ± 2.3 | 3.3 ± 1.5 | 0.002* |
| Consumption opioid dose(mg) | 33.7 ± 18.7 | 23.8 ± 13.4 | 0.1 |
| CRP difference before and 4 hours after surgery(mg/liter) | 20 ± 19.1 | 42. 4 ± 37 | 0.001* |
[Table/Fig-8] shows the mean fatigue score in the MP group was significantly lower than the C group (p = 0.002).
Comparison of fatigue score in Methylprednisolone (MP) and Placebo groups
Discussion
Current study indicated that single high-dose of methylprednisolone 125 mg in combination with analgesic regimen could reduce pain after surgery in patients with stable intertrochanteric unilateral fracture undergoing elective surgery. Overall, pain in three states, rest, hip flexion, and walking, was significantly lower in the MP group than the C group. This finding was similar to Romundstad and Lunna’s studies that showed administration of MP after surgeries as single-dose (125 mg) can significantly reduce post-operative pain in patients [14,23,27,31]. Methylprednisolone belongs to the glucocorticoids group with low power and 125 mg of it equals to 25 mg Dexamethasone and has mild Mineralocorticoid effects. Glucocorticoids are known as anti-inflammatory agents that act as suppressants of Arachnoid acid products through inhibition of Lipocortin-induced phospholipase, and ultimately lead to inhibition of prostaglandins and leukotrienes [32]. Also they prevent production of cytokine that plays a major role in the mechanism of inflammatory pain [33].
The present study supported the above findings and indicated that Methylprednisolone can significantly suppress inflammatory response (CRP) which is also consistent with the finding by Lunn and Bisgaard who indicated Methylprednisolone causes fatigue reduction after surgery which in turn, can justify reduced inflammatory response [22,23,27].
The study revealed that incidence of nausea and vomiting was not statistically significant. However, the above study did not show significant effectiveness of MP on reduced nausea and vomiting [27].
In the current study, the number and amount of Post-operative opioid consumption was lower in the MP group, however this difference was not significant. In the study by Lunn, Methylprednisolone consumption had no effect on the reduced opioid consumption [27]. Also Kardash showed Dexamethasone with dose 40 mg had no significant effect on the reduction of morphine reception in patients after total hip arthroplasty [13]. De Oloveira in a meta-analysis showed that Dexamethasone with dose over 0.1 mg/k was an effective complement in combined (multimodal) strategies for reducing pain and opioid consumption after surgery [34].
One of the strengths of the current study is pain evaluation from different aspects and using Apotel infusion. The limitations of the study include a lack of investigation of methylprednisolone adverse effects due to the lack of access to patient for longer follow-up. Although complications such as wound infection, decreased wound healing, and GI bleeding in frequent dosages or long-term use of glucocorticoids are generally common they have not been observed in the studies on single-dose consumption of these medicines [35].
Conclusion
Single high-dose of methylprednisolone 125 mg (IV) can reduce pain after surgery in patients with intertrochanteric fracture undergoing elective surgery.