Rumpel-Leede Phenomenon in a Hypertensive Lady on Amlodipine
Kandan Balamurugesan1, Stalin Viswanathan2
1 Associate Professor, Department of General Medicine, Indira Gandhi Medical College, Kadhiramam, Pondicherry, India.
2 Assistant Professor, Department of General Medicine, Indira Gandhi Medical College, Kadhiramam, Pondicherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Stalin Viswanathan, Assistant Professor, Department of General Medicine, Indira Gandhi Medical College, Kadhiramam, Pondicherry-605009, India.
Phone: 9894787277,
E-mail: stalinviswanathan@ymail.com
We are describing a 60-year-old hypertensive lady who developed Rumpel-Leede phenomenon following the use of a tourniquet to obtain a blood sample. History revealed that she was on amlodipine therapy and that spontaneous sun-exposure related purpura was often seen since amlodipine was prescribed. Examinations and investigations provided normal results. She refused consent for a skin biopsy. Symptoms resolved after its substitution with enalapril and dihydrochlorothiazide, without any further recurrence.
Calcium channel-blockers, Amlodipine, Purpura, Cutaneous reactions, Skin manifestations
Case Report
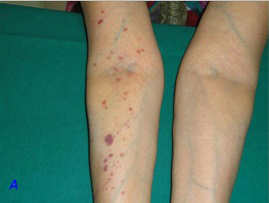
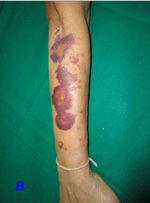
This 60-year-old hypertensive lady for the preceding three months, came to the Out-Patients Department of our hospital for a general check-up. She had lived in north India for her entire life and had moved to our city only 2 weeks ago. She had been prescribed amlodipine 5 mg OD and her compliance and control had been adequate. Her body mass index was 23.83 [height 156 cm and weight 58 kg]. Her systemic examination was within normal limits. She was evaluated and was advised to return with blood sugar and lipid profile results. Following use of a tourniquet on her right arm [in the laboratory] to obtain a blood sample, she had developed multiple petechiae and non-palpable purpura (>40) on her right forearm [Table/Fig-1A]. Ten minutes later, some of them had progressed to ecchymosis [Table/Fig-1B], which gradually resolved over three days. On reviewing her history, she was found to have a history of spontaneous purpura since the previous three months. This had begun 10 days following initiation of amlodipine for controlling her hypertension. There was no history of use of any other drugs. These lesions got worse on sun exposure. There was also a history of gum bleeding when she used a toothbrush and she had resorted to using her fingers. There were no other bleeding complaints or histories which suggested an autoimmune disease. There were no constitutional symptoms. She had been worked up for these complaints and had been told that her blood tests were normal. Complete blood counts, bleeding time, clotting time, prothrombin time, activated partial thromboplastin time, creatinine and blood sugars were normal [Table/Fig-2]. Antinuclear antibody was negative. She refused consent for a skin biopsy. Suspecting calcium channel-blocker (CCB) purpura, enalapril (5 mg) and dihydrochlorothiazide (12.5 mg) were substituted for amlodipine. Her purpura gradually disappeared after a week, without further recurrence.
Immediate development of petechia and purpura on the right arm and forearm after use of tourniquet to obtain a venous sample. Absence of similar lesions on the left upper limb is observed
Increase in purpura and development of ecchymosis on the extensor aspect of forearm 10 minutes after removal of tourniquet
Investigations of patient following onset of purpura
| Investigation | Value | Normal values |
|---|
| Fasting blood sugar | 106 | 70-100 mg/dL |
| Post-prandial sugar | 137 | 140-200 mg/dL |
| Total cholesterol | 188 | <200 mg/dL |
| Triglycerides | 164 | <150 mg/dL |
| LDL cholesterol | 85 | <100 mg/dL |
| HDL cholesterol | 48 | <50 mg/dL |
| Urine microalbumin | 17 | <20 μg/g |
| Hemoglobin | 10.4 | 12-14 g/dL |
| Platelets | 170 | 150-450x103/mm3 |
| International Normalized Ratio | 1.1 | |
| Activated partial thromboplastin time | 28s | 26-39s |
| Clotting time | 2 min 15s | 4-10 min |
| Bleeding time | 1min 35 s | 2-5 min |
| Antinuclear antibody | 0.6 | <1.0 U |
Discussion
Amlodipine is a calcium channel-blocker which acts on cardiac and smooth muscles and which prevents trans-membrane influx of calcium, that causes reduction of peripheral resistance [1]. Skin manifestations which follow amlodipine therapy, that have been reported, include urticaria, alopecia, dermatitis, erythema multiformae, maculopapular rash or an erythematous rash like purpura [1]. The calcium channel blockers (CCBs) include dihydropyridines [amlodipine], phenylalkylamines [verapamil], benzothiazepines [diltiazem] and piperazines [flunarizine]. Side effects which have been ascribed to these drugs include headache, nausea, weight gain, oedema and depression [2]. Cutaneous adverse drug reactions are most commonly associated with diltiazem, among all CCBs [3]. Skin reactions which have been described following use of CCBs include angioedema, exfoliative dermatitis, erythema multiforme, erythema nodosum, rash with photosensitivity, psoriasiform eruptions, urticarial vasculitis, vasculitic leg ulcers, gingival hyperplasia, erythromelalgia, facial telangiectasia, pemphigus foliaceus and nonthrombocytopenic purpura [2]. Serious cutaneous syndromes include Steven-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and hypersensitivity syndrome [2]. Mean age of patients with CCB related cutaneous reactions was 65 years in one study, with the percentage of affected women being higher than that of men [2]. A 2013 study done on usage of calcium channel blockers in the elderly, showed that they were related to chronic eczematous eruptions in this age group [4].
Rare dermatological reactions which occurred during amlodipine therapy included granuloma annulare [1] (that resolved three months after discontinuation), cutaneous hyperpigmentation, TEN [5], longitudinal melanonychia (in an Indian man), [6] erythema multiformae (when nifedipine was substituted) [7], perforating lichenoid eruptions (developing three months after initiation of treatment) [8] and generalized pruritus (one case which was associated with a maculopapular rash) [9]. CCBs in general, can lead to pigmented purpuric dermatosis, purpuric drug eruptions, haemorrhages, purpurae and bleeding, after dermatological surgical procedures [10]. Bleeding manifestations caused by amlodipine are even rarer and those which are reported comprise purpura, haematomas, [11] gum bleeds, haematuria and haematospermia [10]. Haematomas and purpura can occur with or without the occurrence of thrombocytopaenia [11]. There are three reports on amlodipine induced thrombocytopaenia [11]. Kuo et al., presented one case of petechial rash which had occurred without thrombocytopaenia. Non thrombocytopaenic purpura has also been described to occur with use of diltiazem and nifedipine [12]. Cross reactivities may exist among calcium channel blockers. An older age, as in our case, combined with an amlodipine-related increased capillary pressure (caused by relaxation of a pre-capillary sphincter), increased capillary fragility and possible RBC extravasations, may have led to purpura and petechiae. An acute dermal capillary rupture (Rumpel–Leede phenomenon) has been associated with non-invasive blood pressure monitoring, intravenous drug users, Rocky Mountain spotted fever, diabetes, fat embolism and old age [13]. It had been earlier used to assess thrombocytopaenia, but thrombocytopaenia was not contributory in our case. With regards to other CCBs, thrombotic microangiopathy in over-dose of verapamil (+trandalopril) [14] and inhibition of platelet aggregation in diltiazem therapy [10], have been proposed as predisposing causes of bleeding. Though amlodipine has been reported to cause rare cutaneous reactions, there is one description of a cold-induced urticaria which had occurred in an elderly lady, that was controlled, surprisingly with amlodipine [15].
Conclusion
We described a rare, non-fatal adverse skin reaction which had occurred to amlodipine, that had resolved with discontinuation of therapy. An increased venous return which occurs in an elderly hypertensive while using a tourniquet, may contribute to vascular fragility related subcutaneous haemorrhage and this non-drug related effect may have been a limiting feature in this patient. Such reactions may surprise physicians, cause cosmetic embarrassment to fair-skinned patients like ours and lead to unexpected discomfitures to surgeons and dermatologists alike, while they deal with hypertensive patients who are on amlodipine.
[1]. Lim AC, Hart K, Murrell D, A granuloma annulare-like eruption associated with the use of amlodipineAustralas J Dermatol 2002 43(1):24-27. [Google Scholar]
[2]. Knowles S, Gupta AK, Shear NH, The spectrum of cutaneous reactions associated with diltiazem: three cases and a review of the literatureJ Am Acad Dermatol 1998 38(2):201-06. [Google Scholar]
[3]. Cholez C, Trechot P, Schmutz J-L, Bene M-C, Barbaud A, Maculopapular rash induced by diltiazem: allergological investigations in four patients and cross reactions between calcium channel blockersAllergy 2003 58(11):1207-09. [Google Scholar]
[4]. Summers EM, Bingham CS, Dahle KW, Sweeney C, Ying J, Sontheimer RD, Chronic Eczematous Eruptions in the Aging: Further Support for an Association With Exposure to Calcium Channel BlockersJAMA Dermatol 2013 149(7):814-18. [Google Scholar]
[5]. Murthy MB, Murthy B, Amlodipine-induced petechial rashJ Postgrad Med 2011 57(4):341-42. [Google Scholar]
[6]. Sladden M, Mortimer N, Osborne J, Amlodipine-induced longitudinal melanonychia and Hutchison’s signJ Am Acad Dermatol 2005 52(3):P143 [Google Scholar]
[7]. Bewvley AP, Feher MD, Staughton RCD, Erythema multiforme following substitution of amlodipine for nifedipineBMJ 1993 307(6898):241 [Google Scholar]
[8]. Lakshmi C, Srinivas CR, Ramachandran B, Pillai SB, Nirmala V, Perforating lichenoid reaction to amlodipineIndian J Dermatol 2008 53(2):98-99. [Google Scholar]
[9]. Orme S, da Costa D, Drug points: Generalised pruritus associated with amlodipineBMJ 1997 315(7106):463 [Google Scholar]
[10]. Cox NH, Walsh ML, Robson RH, Purpura and bleeding due to calcium-channel blockers: an underestimated problem? Case reports and a pilot studyClin Exp Dermatol 2009 34(4):487-91. [Google Scholar]
[11]. Garbe E, Meyer O, Andersohn F, Aslan T, Kiesewetter H, Salama A, Amlodipine-induced immune thrombocytopeniaVox Sang 2004 86(1):75-76. [Google Scholar]
[12]. Kuo M, Winiarski N, Garella S, Nonthrombocytopenic purpura associated sequentially with nifedipine and diltiazemAnn Pharmacother 1992 26(9):1089-90. [Google Scholar]
[13]. Jeon YS, Kim YS, Lee JA, Seo KH, In JH, Rumpel-Leede phenomenon associated with noninvasive blood pressure monitoring -A case reportKorean J Anesthesiol 2010 59(3):203-05. [Google Scholar]
[14]. Gokel Y, Paydas S, Acikalin A, Bozkurt A, High-dose verapamil + trandolapril-induced thrombotic microangiopathyHaematologia (Budap) 2002 32(3):281-5. [Google Scholar]
[15]. Mahmoudi M, Teuber SS, Cold-induced urticaria controlled by amlodipineJ Allergy Clin Immunol 2000 105(1):S41 [Google Scholar]