Background: Hepatitis C virus (HCV) is transmitted by blood and blood products and it causes a major proportion of transfusion transmitted hepatitis. It can lead to chronic liver disease which has great morbidity and mortality. HCV is responsible for more deaths than Human immunodeficiency virus (HIV). As no vaccine is available and as the treatment is costly and lengthy, with a poor success rate, donor screening remains a very important means of primary prevention of HCV transmission.
Aims and Objectives: This study was conducted to know the prevalence of anti-HCV in healthy voluntary blood donors (VBD) in a semi-urban region of western Maharashtra, India with a special focus on female donors.
Settings and Design: This was an unlinked, anonymous, retrospective study.
Materials and Methods: During January 2006 to December 2012, sera of 17976 VBD, which comprised of 16972 (94.41%) males and 1004 (5.59%) females, were tested for presence of anti-HCV antibody (anti-HCV) by using a 3rd generation ELISA test. Data was statistically analyzed by using Chi-Square for linear trends (Extended Mantel-Haenszel test). - 0.72732.
Results and Conclusion: Thirty six donors (0.2%) were positive for anti-HCV. Seroprevalence in males was 0.21%, while that in females was 0%. The positivity of anti-HCV remained stable over the tenure of this study (Chi-Square for linear trends - 0.72732). This region has a lower prevalence of anti-HCV as compared those seen in other states of India. Zero prevalence in women indicated that encouraging women to undergo blood donations would still reduce the transmission of HCV. Detection can be improved by doing better tests like HCV RNA detection and further prevention of HCV transmission can be enhanced.
Voluntary blood donors, Blood transfusion, Hepatitis C, HCV, Anti-HCV
Introduction
Being formerly called as the Non-A Non-B hepatitis virus, the hepatitis C virus (HCV) was first detected in 1989 in experimental animals by isolation of cDNA from blood [1]. HCV is a 55 nm spherical enveloped RNA virus. It belongs to family, Flaviviridae, and it has been classified into a distinct genus, Hepacivirus. Its genome is 9.6 kb long, single stranded, positive sense RNA. It codes for several structural and functional proteins of the virus. Six major genotypes and more than 80 subtypes of HCV have been identified [2]. Initially, it was thought to cause an infection of only minor importance, affecting only drug abusers and blood product recipients in developed countries. It has now been proved that HCV is a global concern, as it causes many health problems. HCV is responsible for a significant proportion of post transfusion hepatitis cases. It is one of the leading causes of chronic liver disease in the entire world. HCV infection, particularly in its chronic form, is associated with great morbidity and mortality. Presently, it has been noticed that hepatitis C is responsible for more deaths than HIV [3].
The high risk populations for HCV infection include injectable drug users (IDU), blood transfusion recipients, sexually promiscuous individuals, haemodialysis patients, HIV positive persons, kidney transplant recipients and prisoners. Among all these, the IDU are highest in number, and this is the primary mode of HCV transmission in developed countries. Though the transfusion of blood and blood products was a leading cause of transmission of HCV, after the introduction of screening of blood units for HCV in blood banks in 1990, such a transmission has decreased in most of the developed countries. Unfortunately, the incidence of transfusion related hepatitis C is still higher in developing countries like India [4].
The estimated figures of HCV infection are quite alarming - three to four million individuals newly acquire HCV infection every year, 170 million have chronic infection with a risk of cirrhosis and malignancy and yearly, 350,000 deaths are caused by HCV related causes. According to WHO, 12 million Indians are suffering from hepatitis C. Prevalence of HCV in healthy blood donors represents prevalence of carrier state in the population. High rate of anti-HCV antibody (anti-HCV) positivity, which is seen in individuals who are transfused multiple times, is an indicator of risk of contracting HCV by blood transfusion. The prevalence of anti-HCV in blood donors has been reported from various countries and from various parts of India. Though in other countries, IDU is the major mode of HCV transmission, in India, blood transfusion is primarily responsible for it. As no vaccine is available and as the treatment is costly and lengthy, with a poor success rate, donor screening remains a very important means of primary prevention of HCV transmission [5].
The present study was conducted to determine the prevalence of HCV antibodies in voluntary blood donors (VBD), with a special focus on female donors and to know the impact of a mandatory screening.
Materials and Methods
Study period - The study period extended over 7 years, from January 2006 to December 2012. During this period, 17976 VBD who visited the blood bank of Dr. D.Y. Patil Medical College, Hospital and Research Centre, Pimpri, Pune, Maharashtra, India were tested for presence of anti-HCV. Those who showed presence of anti-HCV were referred as seropositive. The data was analyzed to determine the seroprevalence of anti-HCV.
Sample collection and laboratory testing - 3 ml of blood from each donor was collected in a plain bulb, it was allowed to clot and separated serum was used for the test.
The detection of anti- HCV in human serum was achieved by using a commercially available 3rd generation ELISA kit, the HCV Microlisa (J. Mitra and Co.). This test utilizes a combination of both structural (Core, E1 and E2) and non-structural antigens (NS3, NS4 and NS5) of HCV, which increases specificity of the test.
Ethical issues - This was an unlinked and anonymous, retrospective observational study. Since HCV testing of donors is part of the statutory requirement in blood banks, no separate consent was required. HCV positive units of blood were discarded. All personal details of the individuals who were tested were removed from the blood samples, to delink HCV testing from the identity of the person. This study was approved by the institutional ethics committee.
Statistical Analysis
Data were summarized by using percentages. Year wise anti-HCV prevalence among the blood donors was calculated. The data was analyzed by using Chi-Square for linear trend (Extended Mantel-Haenszel test).
Results
A total number of 17976 VBD who comprised 16972 (94.41%) males and 1004 (5.59%) females were studied during a seven year period. [Table/Fig-1] shows the annual gender distribution of donors and their HCV seropositivities.
Annual gender distribution of donors and HCV seropositivity
| Year | Male Donors | Female Donors | Total donors | HCV seropositive (%) |
|---|
| Total | HCV seropositive (%) | Total | HCV seropositive (%) |
|---|
| 2006 | 1816 | 5 (0.27) | 118 | 0 (0) | 1934 | 5 (0.26) |
| 2007 | 2254 | 1 (0.04) | 100 | 0 (0) | 2354 | 1(0.04) |
| 2008 | 2261 | 5 (0.24) | 78 | 0 (0) | 2339 | 5(0.21) |
| 2009 | 1920 | 5 (0.26) | 78 | 0 (0) | 1998 | 5(0.25) |
| 2010 | 1369 | 0 (0) | 101 | 0 (0) | 1470 | 0 (0) |
| 2011 | 3355 | 10 (0.30) | 277 | 0 (0) | 3632 | 10(0.27) |
| 2012 | 3997 | 10 (0.27) | 252 | 0 (0) | 4249 | 10(0.24) |
| Total | 16972 | 36 (0.21) | 1004 | 0 (0) | 17976 | 36 (0.20) |
A total of 36 (0.21%) VBD were positive for anti-HCV in their sera. All the seropositives were males, which showed a zero seroprevalence of HCV in females. The overall prevalence of anti-HCV was found to be 0.2%. As can be seen from [Table/Fig-1], the positivity of anti-HCV has remained stable over this tenure and there has been no significant rising or decreasing trend [Chi-Square for linear trend (Extended Mantel-Haenszel test) - 0.72732]. In year 2010, not a single VBD was found to be HCV seropositive.
Discussion
This study has illustrated overall trend of the epidemiology of this infection in the community. Amongst 17976 VBD who were studied, 16972 (94.41%) were males; whereas 1004 (5.59%) were females. The male preponderance may be caused by factors affecting women: fear of blood donation, lower levels of education and cultural restrictions on social movement of women in India. In this retrospective study, we evaluated the seroprevalence of the anti-HCV among the VBD in Pimpri region of Pune, Maharashtra, irrespective of their ages. The given data also helped us in evaluating the seroprevalence of Hepatitis C infection amidst male and female donors. This seven year’s study revealed the prevalence of HCV infection in a community which resided in semi-urban area of western Maharashtra, India.
The overall anti-HCV positivity amongst the VBD was 0.2% and all of them were males, which showed a zero percent prevalence in females. Similar findings were reported from Andhra Pradesh and Orissa [6,7]. A report from West Bengal [8] showed 0.59% seroprevalence in female VBD. The higher prevalence in males which was seen, may be because more males opt for blood donation and hence, more males are tested. Also, the number of males who receive transfusions of blood and blood products is more. Thirdly, males indulge into risk behaviours much more than females. As per WHO fact sheet of 2013, globally, approximately 30% of the donated blood comes from women donors. Out of 104 countries which report to WHO, 18 countries receive less than 10% of the donations from females [9]. Gender wise percent prevalence of anti-HCV in other countries has been shown in [Table/Fig-2].
Gender wise percent prevalence of anti-HCV in VBD from various countries
| Country (Reference No.) | % prevalence in VBD |
|---|
| Male | Female |
|---|
| Tanzania [10] | 1.5 | 1.1 |
| USA [11] | 0.88 | 0.056 |
| Jorden [12] | 0.793 | 0.097 |
| Manila [13] | 0.35 | 0.10 |
| Nepal [14] | 0.19 | 0.099 |
| Philippines [15] | 0.104 | 0.34 |
| Present study | 0.21 | 0.20 |
A community study done in China showed anti-HCV prevalence to be 1.16% in males and 2.01% in females [16]. It is notable that in Philippines and China, anti-HCV seroprevalence is higher in females than in males [15,16]. Lowest prevalence in females was observed in USA [11].
In countries like USA and China, it was observed that number of female VBD was equal or more than male VBD [11,16]. But in all other studies, female VBD were very small in number and this prevented us from getting a very correct idea about anti-HCV prevalence in female donors [10,12–15].
Apart from very low number of female donors, low prevalence seen in females may be caused by comparatively less indulgence of females in risk behaviours. Also, the number of females who receive blood transfusions is small in most of the developing countries. Hence, true prevalence in females can be determined by studying a large sample size. Nevertheless, with the presently available data which shows mostly very low anti-HCV prevalence in females, it may be said that women donors can be safer than men and that they should be encouraged to donate blood, to ensure safety of blood transfusion and to prevent wastage of blood units.
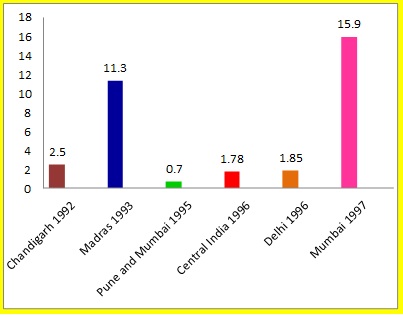
In India, mandatory screening for HCV started [17]. The prevalence of HCV has been showing a downfall since then. The percentage seroprevalence of anti-HCV in VBD in various places in India before year 2000 was reported to be between 0.7-15.9 [Table/Fig- 3] and it was more than one in most of the reports [18–23]; while that after year, 2000, it had ranged from 0.13 to 1.09 [Table/Fig-4] and it was less than one in most of the reports [6,24–30]. This clear reduction was a very good sign and it reflected the effects of control measures. [Table/Fig-3] shows prevalence of anti-HCV in VBD in India. Some studies done in north India in this decade showed seroprevalence which was at the upper end of the range. Lowest prevalence of 0.13% was reported from Vellore. Present study showed that anti-HCV seroprevalence in this area was lower (0.2%) and that it was closer to that which was reported by Gowri et al., [30]. Seroprevalence in Maharashtra in all donors was 0.7% [20]. A study done on rural donors by Sonwane et al., [31] showed zero percent seroprevalence, while another recent study done in rural Maharashtra showed a prevalence of 0.74% [29]. This may be a side effect of rapid urbanization of villages, which had exposed people to risk factors for HCV transmission. A significantly lower seroprevalence which was observed in present study, may have been caused by stringent implementation of mandatory HCV screening in the blood banks since 2002. Maharashtra state as such and the region where this study was conducted are progressive regions of the country, with good and widespread education. As HIV and HCV share the same modes of transmission, the rigorous health education and measures of controlling HIV, such as wide use of condoms, also may have contributed to limiting spread of HCV.
Anti-HCV prevalence in India before year 2000 [18–23] X axis- Place of study and year; Y axis- Percent prevalence of anti-HCV
Prevalence of anti-HCV in VBD in India after year 2000 [24,25,6,26,27,29,30] X axis-Place of study and year; Y axis- Percent prevalence of anti-HCV
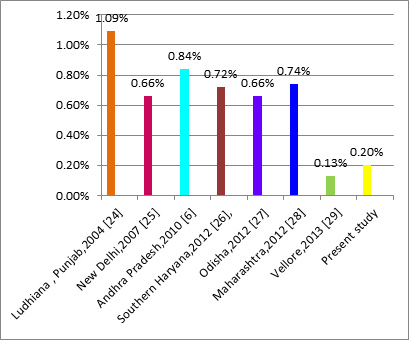
Basically, the blood transfusion services in India are primarily fragmented, disorganized and hospital based, which is aggravated by the lack of a comprehensive and systematic screening of donated blood and the predominance of replacement donors. As can be seen in [Table/Fig-4], in India, rate of anti-HCV positivity ranges from 0.13-1.09%.HCV is globally distributed, with anti-HCV prevalence among donors ranging from 0.3 to 0.5% throughout the world [32]. Hence, in developing countries like India, voluntary donation of blood by such individuals and lack of current blood screening practices are really burgeoning threats to the recipients. The availability of safe blood can only be achieved by vigorous screening of blood donors and donated blood.
Presently, donor screening for HCV infection is based mainly on detection of specific antibody in serum. This does not detect individuals in window period, which is much longer for HCV infection, approximately 66 days, due to delayed antibody production [33,34]. Thus, an antibody based detection fails to diagnose the infection during this period. However, viral particles become detectable earlier, in approximately one week. Based on this, nucleic acid testing (NAT) can be used as an alternative [35]. A recent report from New Delhi showed that 38 sera from VBD were positive for HCV by NAT, while of these 38, only 35 were positive for anti-HCV by ELISA. Relying only on ELISA would result in non-diagnosis of few cases [36]. With component separation in blood banks, each undiagnosed infected donor can infect 2-3 recipients. The projected magnitude of blood and blood component recipients who are infected this way can be frightening. High costs, a greater turnover time and a need of high technical expertise are the limiting factors in the way of the generalized use of NAT, more so in developing countries. However, it is worth considering, as the window period for HCV is reduced by NAT, and as the benefits of an early detection may outweigh the risks which are involved. Though NAT is the future of HCV diagnosis in India, presently, a very stringent donor screening for anti-HCV is very important, to ensure blood safety.
Conclusion
Our study highlighted that overall anti-HCV prevalence in VBD in this semi-urban region of Maharashtra was 0.2% and that in women, it was zero percent. Encouraging women for blood donations can be considered as one positive step which is taken for ensuring blood safety. Technologically advanced laboratories require highly qualified and highly skilled technicians, colossal amount of money and well equipped laboratories, which unfortunately, are difficult to build up in developing countries with limited resources. A public private partnership which is made to share resources and skilled technicians can become useful for tackling this problem. Rigorous health education, strict monitoring of blood banks and implementation of law regarding donor screening, would go a long way in prevention of transfusion transmitted hepatitis and they will reduce the economic burden on government.
[1]. Choo QL, Kuo G, Weiner AJ, Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genomeScience 1989 244:359-62. [Google Scholar]
[2]. Ashfaq UA, Javed T, Rehman S, Nawaz Z, Riazuddin S, An overview of HCV molecular biology, replication and immune responsesVirol. J 2011 8:161 [Google Scholar]
[3]. KN Ly, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD, The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007Ann Intern Med 2012 156:271-8. [Google Scholar]
[4]. Alter MJ, Epidemiology of hepatitis C virus infectionWorld J Gastroenterol 2007 13(17):2436-41. [Google Scholar]
[5]. World Health Organization. Hepatitis C, 2003. Available from http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index4.html [Google Scholar]
[6]. Bhawani Y, Rao PR, Sudhakar V, Seroprevalence of transfusion transmissible infections among blood donors in a tertiary care hospital of Andhra PradeshBiology and Medicine 2010 2:45-8. [Google Scholar]
[7]. Misra S, Chayani N, Sarangi G, Mallick B, Pati SB, Seroprevalence of anti-HCV antibody in and around Cuttack, OrissaIndian J Med Microbiol 2002 20:40-1. [Google Scholar]
[8]. Das BK, Gayen BK, Aditya S, Chakrovorty SK, Datta PK, Joseph A, Seroprevalence of Hepatitis B, Hepatitis C and human immunodeficiency virus among healthy voluntary first-time blood donors in KolkataAnn Trop Med Public Health 2011 4:86-90. [Google Scholar]
[9]. WHO Fact Sheet-Blood safety and availability. Available from http://www.who.int/mediacentre/factsheets/fs279/en/index.html [Google Scholar]
[10]. Matee MI, Megesa PM, Lyamuya EF, Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis infections among blood donors at the Muhimbili National Hospital in Dar Es Salaam, TanzaniaBMC Public Health 2006 6:1-6. [Google Scholar]
[11]. Murphy EL, Fang J, Tu Y, Cable R, Hillyer CD, Sacher R, Hepatitis C Virus Prevalence and Clearance among US Blood Donors,2006-2007:Associations with Birth Cohort, Multiple Pregnancies, and Body Mass IndexJ Infect Dis 2010 202:576-84. [Google Scholar]
[12]. Gani FA, Prevalence of HBV, HCV and HIV-1, 2 infections among blood donors in Prince Rashed Ben Al-Hassan Hospital in North Region of JordonInt J Biol Med Res 2011 2:912-6. [Google Scholar]
[13]. Yanase Y, Ohida T, Kaneita Y, Agdamag DMD, Leaño PSA, Gill CJ, The prevalence of HIV,HBV and HCV among Filipino blood donors and overseas work visa applicantsBull World Health Organ 2007 85:131-7. [Google Scholar]
[14]. Tiwari BR, Ghimire P, Kandel SS, Rajkarnikar M, Seroprevalence of HBV and HCV in blood donors: A study from regional blood transfusion service of NepalAsian J Transfus Sc 2010 4:91-3. [Google Scholar]
[15]. Rodenas JG, Bacasen LC, Que ER, The prevalence of HBsAg (+) and anti-HCV(+) among healthy blood donors at east avenue medical center, Quezon cityPhilippines Journal of Gastroenterology 2006 2:64-70. [Google Scholar]
[16]. Li D, Long Y, Wang T, Xiao D, Zhang J, Guo Z, Epidemiology of hepatitis C virus infection in highly endemic HBV areas in ChinaPLOS one 2013 8:1-5. [Google Scholar]
[17]. Mukhopadhyaya A, Hepatitis C in IndiaJ Biosci 2008 33:465-73. [Google Scholar]
[18]. Sood G, Chauhan A, Sehgal S, Agnihotri S, Dilawari JB, Antibodies to hepatitis C Virus in blood donorsInd J Gastroenterol 1992 11:44 [Google Scholar]
[19]. Sumathy S, Vallimmai T, Thyagarajan SP, Malathy S, Madanagopalan N, Sankaranarayanan V, Prevalence of hepatitis C virus infection in liver diseases, renal diseases and voluntary blood donors in south IndiaIndian J Med Microbiol 1993 11:291-7. [Google Scholar]
[20]. Arankalle VA, Chadha MS, Jha J, Amrapurkar DN, Banerjee K, Prevalence of anti-HCV antibodies in western IndiaIndian J Med Res 1995 101:91-3. [Google Scholar]
[21]. Jaiswal SPB, Sepaha A, Pandit CS, Chitnis DS, Naik G, Artwani KK, Prevalence of anti-HCV antibodies in central IndiaIndian J Med Res 1996 104:177-81. [Google Scholar]
[22]. Panigrahi AK, Acharya SK, Jameel S, Panda SK, Genotype determination of hepatitis C virus from northern India: identification of a new subtypeJ Med Virol 1996 48:191-8. [Google Scholar]
[23]. Gosavi MS, Shah SK, Shah SR, Pal RB, Saldanha JA, Banker DD, Prevalence of hepatitis C virus (HCV) infection in MumbaiIndian J Med Sci 1997 51:378-85. [Google Scholar]
[24]. Gupta N, Kumar V, Kaur A, Seroprevalence of HIV, HBV, HCV and Syphilis in voluntary blood donorsIndian J Med Sci 2004 58:255-7. [Google Scholar]
[25]. Pahuja S, Sharma M, Baitha B, Jain M, The prevalence and the trends of the markers of the hepatitis C virus, the hepatitis B virus and the human immunodeficiency virus in the Delhi blood donors: A hospital based studyJpn J Infect Dis 2007 60:389-91. [Google Scholar]
[26]. Kochhar AK, Duggal G, Trend in seroprevalence of hepatitis C virus infection among blood donors of southern HaryanaJARBS 2012 4:219-22. [Google Scholar]
[27]. Poddar N, Lenka PR, Chayani N, Mohanty S, Mallick B, Pattnaik D, Seroprevalence of hepatitis-c virus in blood donors and high risk individualsJ Evol Med and Dent Sci 2012 1:959-63. [Google Scholar]
[28]. Kaur H, Manjari M, Thaman RG, Singh G, Prevalence of markers of hepatitis c virus among the blood donorsJ Clin Diagn Res 2012 6:959-62. [Google Scholar]
[29]. Giri PA, Deshpande JD, Phalke DB, Karle LB, Seroprevalence of transfusion transmissible infections among voluntary blood donors at a tertiary care teaching hospital in rural area of IndiaJ Fam Med Primary Care 2012 1:48-51. [Google Scholar]
[30]. Gowri V, Chandraleka C, Vanaja R, The current seroprevalence of Hepatitis C virus in a tertiary care centre in Vellore, Tamil NaduIndian J Comm Med 2012 37(2):137 [Google Scholar]
[31]. Sonwane BR, Birare SD, Kulkarni PV, Prevalence of seroreactivity among blood donors in rural populationIndian J Med Sci 2003 57:405 [Google Scholar]
[32]. WHO-HepatitisC.2002.Available from http://www.who.int/csr/disease/hepatitis/Hepc.pdf [Google Scholar]
[33]. Kucirkaa LM, Sarathya H, Govindanb P, Wolfa JH, Ellisona TA, Hartc LJ, Risk of window period hepatitis-C infection in high infectious risk donors: Systematic review and meta-analysisAm J Transplant 2011 11:1188-1200. [Google Scholar]
[34]. Rehermann B, Hepatitis C virus versus innate and adaptive immune responses: A tale of coevolution and coexistenceJ Clin Invest 2009 119:1745-54. [Google Scholar]
[35]. Singer AL, Kucirka LM, Namuyinga RHC, Subramanian AK, Segev DL, The high risk donor: Viral infections in solid organ transplantationCurrent Opinion Org Transpl 2008 13:400-4. [Google Scholar]
[36]. Chatterjee K, Coshic P, Borgohain M, Premchand Thapliyal RM, Chakroborty Sunder S, Individual donor nucleic acid testing for blood safety against HIV-1 and hepatitis B and C viruses in a tertiary care hospitalNatl Med J India 2012 25:207-9. [Google Scholar]