The frequency of invasive, opportunistic mycoses has increased significantly over the past 2 decades. In the immune-compromised host, many fungi, including species of fungi typically considered non-pathogenic, have the potential to cause serious morbidity and mortality. Here we report a rare case of mixed fungal infection of the lung with Candida albicans and Aspergillus fumigatus in a patient on prolonged steroid therapy.
Case Report
A 53-year-old female, from Ananthapur (Andhra Pradesh), was admitted to Narayana Hrudayalaya Hospital, Bangalore, India in the month of February 2013, with history of loose stools, vomiting since 3-4 days and loss of consciousness since that day morning. Patient was intubated immediately due to low Glasgow Coma Scale (GCS). She was treated for the same in another hospital and was referred to our hospital for further management. She was a known case of type 2 diabetes mellitus, coronary artery disease with anterior wall MI, LV dysfunction, hypothyroidism and adrenocortical insufficiency and was on medications for all these conditions since one year.
Investigations – Chest X-ray of patient showed features of pulmonary edema. Total count was 3,500, Neutrophils- 94%, Liver Function Test and Renal Function Test were normal, Procalcitonin 8.4, Stool routine showed occult blood, no ova and cyst. Stool was also sent for culture. Serology for HIV was non-reactive. She was managed medically for pulmonary oedema and started on meropenem and vancomycin in view of infection.
Her endotracheal aspiration was sent next day for culture and sensitivity which grew multi-drug resistant Klebsiella pneumonia. Blood cultures were sent which did not yield any growth. Stool did not show any growth of pathogens. Antibiotics were changed based on sensitivity pattern.
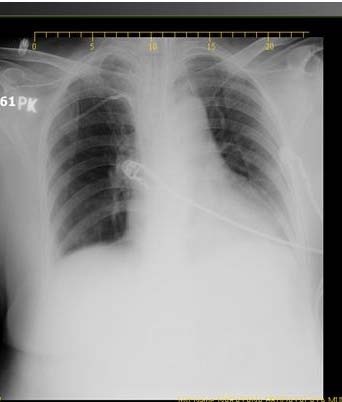
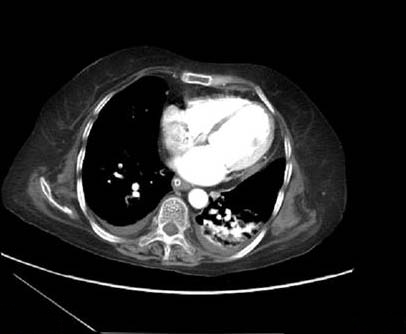
After 4 days, a repeat chest X-ray was taken in view of desaturation showed patch in the left lung [Table/Fig-1]. A CT scan showed thin walled cavity in the left lower lobe of the lung [Table/Fig-2] with pulmonary edema and in the abdomen there was diffuse large bowel thickening. Due to these radiological findings, bronchoscopy was planned. On bronchoscopy necrotic mucosa was seen at the lower end of the trachea extending to the left lower lobe. It was filled with necrotic material and biopsy was taken which was sent for histopathological examination. Bronchioalveolar lavage was sent for culture and sensitivity test, Potassium hydroxide (KOH) preparation and Cytomegalovirus (CMV) qualitative Polymerase chain reaction (PCR).
Chest X-ray showing a patch in left lung
CT scan of chest showing pathology on left lung
The sample was streaked on Blood agar, Chocolate agar and incubated at 37°C for 48 hours and Sabouraud Dextrose Agar (SDA) which was incubated at both 37°C and 25°C for a week.
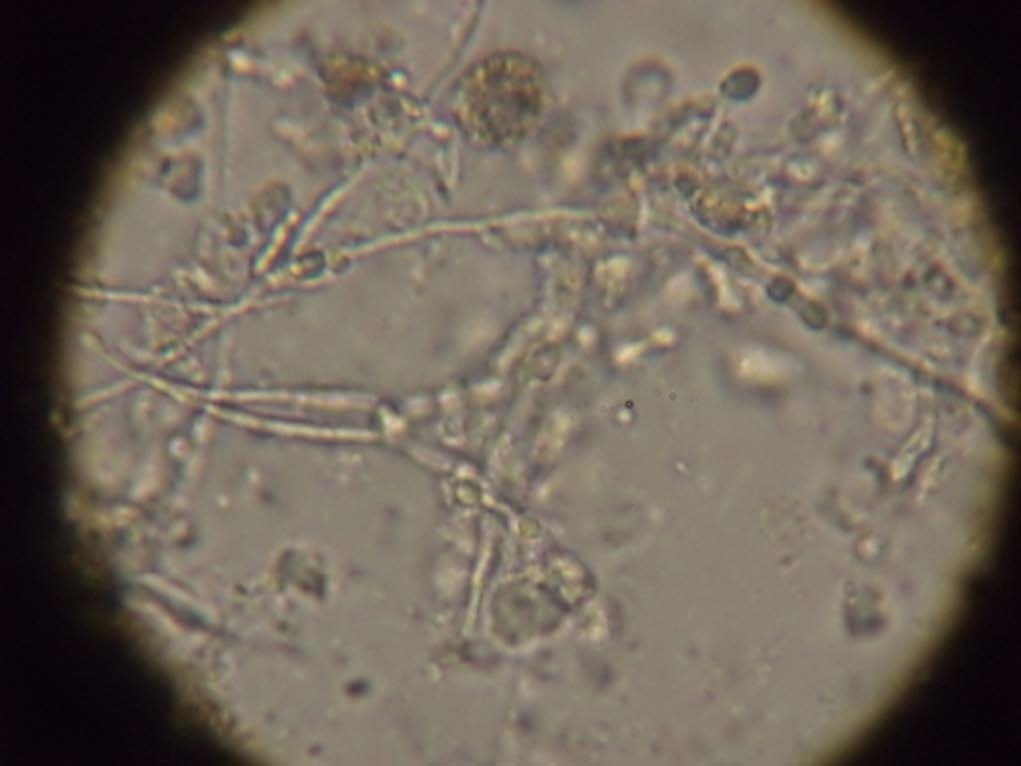
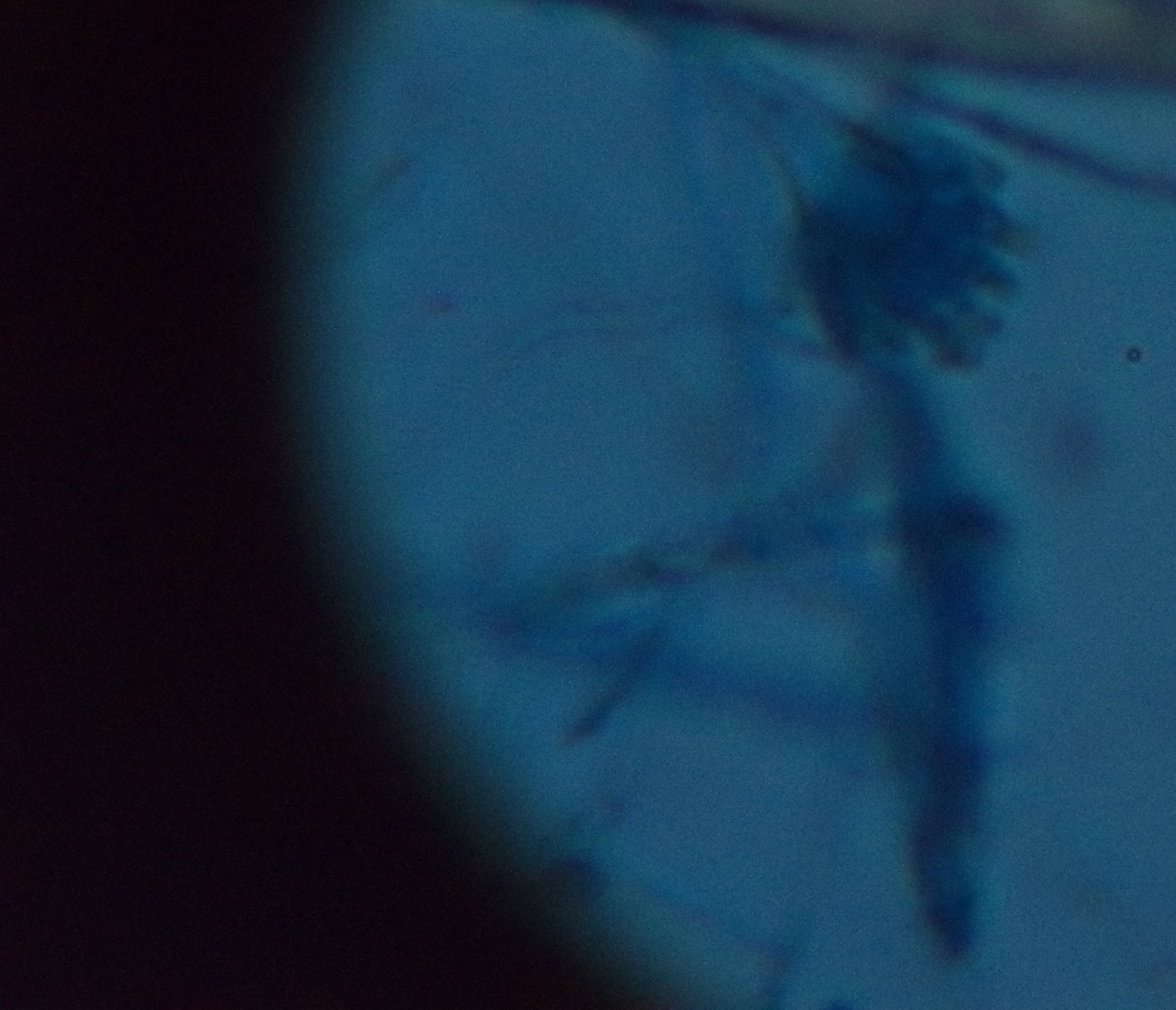
On KOH mount, there were septate hyphae with acute angle branching and oval budding yeast cells seen [Table/Fig-3]. On Gram stain there were few inflammatory cells with oval budding yeast cells with pseudohyphae and septate hyphae seen [Table/Fig-4]. The patient was started on voriconazole and micafungin.
KOH preparation of broncho alveolar lavage showing septate hyphae
Gram stain of bronchoalveolar lavage showing septate hyphae and oval budding yeast cell
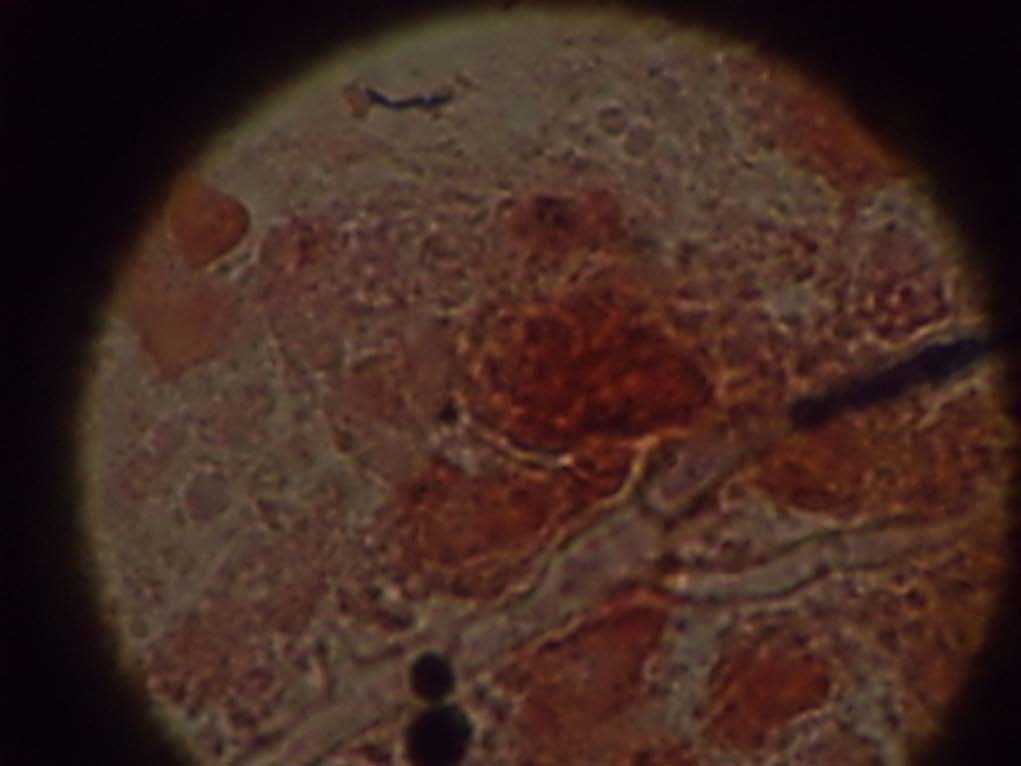
Histopathological examination of the biopsy sent, showed necroinflammatory slough with fungal structures consisting of yeast cells with pseudohyphae and acute angle branching hyphal structures, suggestive of mixed Candidal and Aspergillus invasive fungal disease. After 48 hours there were creamish colonies on SDA resembling yeast, on Gram stain it showed oval budding yeast cells. The yeast like colonies were processed in Vitek 2 for further identification and sensitivity. It was identified as Candida albicans sensitive to amphotericin B(MIC- 0.5), flucytosine, fluconazole
(MIC <1) and voriconazole(MIC <0.12), caspofungin (MIC <0.25)
Patient remained on ventilator support with antibiotics and antifungal agents. The clinical condition remained the same.
After 4 days, there were white velvety colonies resembling a mould in the tube incubated at 370C. The whitish colonies turned green with pale yellow reverse on further incubation and later a Lactophenol cotton blue (LPCB) mount was performed which revealed septate hyphae, terminal vesicles which support a single row of phialides on the upper two-thirds of the vesicle [Table/Fig-5].
LPCB mount showing Apergillus fumigates
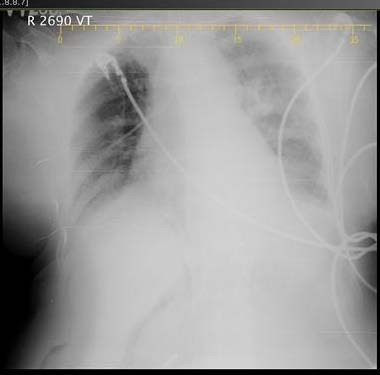
Three days later her repeat chest X-ray showed patch on both right and left side [Table/Fig-6]. A repeat bronchoscopy was performed which revealed necrotic material similar to the previous study. The sample was sent for microbiological and histopathological examination, the results of which were similar to the previous sample. A colonoscopy was performed and biopsy taken which was sent for histopathological examination. Histopathological examination of biopsy showed no evidence of fungal infection.
Chest X-ray showing deterioration in the picture
But next day she developed metabolic acidosis and decreased cardiac output. She had persistant hypotension later and succumbed due to cardiac arrest.
Discussion
The frequency of invasive, opportunistic mycoses has increased significantly over the past two decades. Patients who are at risk for the development of serious fungal infections, including patients undergoing blood and marrow transplantation (BMT), solid-organ transplantation, patients with Acquired immunodeficiency syndrome (AIDS) neoplastic disease, advanced age, patients receiving immunosuppressive therapy and premature Infants [1].
The human airway is continuously open to the non-sterile environment where fungal spores have the potential to reach lung tissue and produce disease. In the immune-compromised host, many fungi have the potential to cause serious morbidity and mortality [2]. The widespread implementation of antimicrobial prophylactic regimens for a variety of immune-suppressed patient groups has rendered the host at greater risk for colonization with more resistant fungal species, enhancing the increase of invasive fungal infection in these already immune-suppressed patients [3]. In our case the patient was a known case of adrenocortical insufficiency and was on steroids for the last 1 year, which could be the cause of immunodeficiency.
The opportunistic yeasts of the Candida species are endogenous flora that can gain access to the bloodstream, usually through the bowel [4]. Two forms of Candida pneumonia have been reported: primary pneumonia, which follows aspiration of Candida laden oropharyngeal secretions, and pneumonia secondary to hematogenously disseminated Candidiasis, especially in immunocompromised hosts [5]. The debate about the diagnosis of pulmonary Candidiasis, the definite diagnosis of pulmonary Candidiasis still rests on histologic demonstration of the yeast in lung tissue with associated inflammation [6]. To rule out dissemination of Candida from the large intestine, a colonoscopy was performed and biopsy taken. But there was no histopathological evidence of fungal infection.
The spores of Aspergillus species enter the body commonly through the sinuses or the respiratory tract. In the early stages of the disease the chest radiograph may be normal. As the disease progresses, a nodular appearance or patchy consolidation may be evident [4]. Similarly in our case in the chest X-ray taken on admission did not show any patch or infiltrate. A patch was seen on the left lung only four days after admission.
The definition for proven Aspergillosis requires histopathological documentation of infection and a positive result of culture of a specimen from a normally sterile site. Aspergillus fumigatus is the most common species recovered from cases of invasive Aspergillosis [7]. Similarly in our case we have isolated Aspergillus fumigates from the culture along with Candida albicans. This is a definite proven case of mixed infection with Candida and Aspergillus due to both histopathological and culture evidence.
Aspergillus fumigatus is inhibited by Candida spp. in culture and the inhibitory effect is fungistatic and not fungicidal [8]. In our culture there was a delay in growth of Apergillus most probably due to the inhibition of growth by Candida spp.
Other than Aspergillus, angioinvasive molds, such as Fusarium and Zygomycetes species, are increasingly encountered in severely immunocompromised hosts [9]. Many of these emerging fungi are resistant to antifungal agents, and despite advances in antifungal therapy, Invasive fungal infections caused by them have high mortality [10].
Simultaneous infections with multiple fungi may be misinterpreted as monomicrobial infections by current diagnostics with ramifications for the choice of antimicrobial agents that may impact patient outcomes. The application of molecular methods on tissue samples may be useful to decipher the aetiology of mixed fungal infections. The combination of broad-range PCR with sequence analysis and FISH applied on tissue samples is a powerful approach to identify the aetiology of invasive fungal infections, including mixed infections [11].
As per the Infectious diseases society of America (IDSA) guidelines for treatment of invasive pulmonary aspergillosis, voriconazole is the recommended drug for primary therapy and alternative drugs are amphotericin B, caspofungin, micafungin and itraconazole [7]. Candida should be treated with antifungal agents, selecting one of the following agents: fluconazole, an amphotericin B formulation, an echinocandin, voriconazole, or the combination regimen of fluconazole and amphotericin B [5].
Inspite of all the appropriate antibiotic and antifungal agents the patient succumbed probably due to the underlying comorbid conditions, resistance of the fungi to the antifungal agents and due to mixed fungal infections which have a higher mortality rate.
Conclusion
Immunocompromised individuals are highly susceptible to the fungal infections. High rate of mortality and morbidity is noticed in patients with mixed fungal infection. Hence high clinical suspicion and appropriate antimicrobial therapy needs to be initiated in immunocompromised patients.
[1]. Michael AP, Peter GP, John RW, Invasive Fungal Pathogens:Current Epidemiological TrendsClinical Infectious Diseases 2006 43:S3-S14. [Google Scholar]
[2]. Jay BV, John R, Rare and Emerging Fungal Pulmonary InfectionsSemin Respir Crit Care Med 2008 29(2):121-31. [Google Scholar]
[3]. Andrew HL, The Changing Spectrum of Fungal Infections In Pulmonary and Critical Care PracticeProceedings of the American Thoracic Society 2010 7(3):163-68. [Google Scholar]
[4]. Ahmad SS, Abdullah WA, Wastie ML, Imaging features of fungal infection in immuno-suppressed patients in a local ward outbreakBiomed Imaging Interv J 2006 2(2):1-8. [Google Scholar]
[5]. Andrew HL, Kenneth SK, George AS, Neil MA, John EB, Antonino C, Treatment of Fungal Infections in Adult Pulmonary and Critical Care PatientsAm J Respir Crit Care 2011 183:96-128. [Google Scholar]
[6]. Mustafa el-Ebiary AT, Neus F, Jorge Puig de la B, Julia G, Josep R, Dolores DB, Carmen H, Significance of the Isolation of Candida Species from Respiratory Samples in Critically Ill, Non-neutropenic PatientsAmerican Journal of Respiratory and Critical Care Medicine 1997 156(2):583-90. [Google Scholar]
[7]. Thomas JW, Elias JA, David WD, Raoul H, Dimitrios PK, Kieren AM, Pulmonary fungal infections in immunocompromised patients: incidence and risk factorsClin Infect Dis 2002 46(3):327-60. [Google Scholar]
[8]. Randhawa HS, Sandhu RS, Kowshik T, In vitro inhibition of Aspergillus fumigatus by Candida species, especially C.Albicans and CglabrataCurrent Science 2002 82(7):860-65. [Google Scholar]
[9]. Hisham W, Mylene TT, Xiudong L, Dimitrios PK, Edith M M, Reversed Halo Sign in Invasive Pulmonary Fungal InfectionsClinical Infectious Diseases 2008 46(11):1733-37. [Google Scholar]
[10]. Sharon CAC, Christopher CB, Tania CS, Monica AS, Pneumonia and Lung Infections Due to Emerging and Unusual Fungal PathogensSemin Respir Crit Care Med 2011 32(6):703-16. [Google Scholar]
[11]. Rickerts V, McCormick Smith I, Mousset S, Kommedal O, Fredricks DN, Deciphering the aetiology of a mixed fungal infection by broad-range PCR with sequencing and fluorescence in situ hybridisationMycoses 2013 56(6):681-86. [Google Scholar]