Male Breast Cancer: Presenting as Synchronous, Large, Bilateral Masses
Sunil Vitthalrao Jagtap1, P. G. Chougule2, Wasim Khatib3, Dhirajkumar B. Shukla4, Swati Sunil Jagtap5
1 Associate Professor, Department of Pathology, Krishna Institute of Medical Sciences University, Karad, Maharashtra, India.
2 Professor, Department of Surgery, Krishna Hospital and Medical Research Center, Karad, Maharashtra, India.
3 Assistant Lecturer,Department of Pathology, Krishna Institute of Medical Sciences University, Karad, Maharashtra, India.
4 Assistant Lecturer, Department of Pathology, Krishna Institute of Medical Sciences University, Karad, Maharashtra, India.
5 Associate Professor, Department of Physiology, Krishna Institute of Medical Sciences University, Karad, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sunil Vitthalrao Jagtap, Associate Professor, Department of Pathology, Krishna Institute of Medical Sciences University, Karad, Maharashtra-415110, India.
E-mail: drsvjagtap@gmail.com
Male breast cancer is a very rare neoplasm which accounts for 1% of all breast cancers. A 70-year-old male presented with a rapidly growing, bilateral breast masses with large size, surface ulceration and bloody discharge. Synchronous bilateral breast cancer was diagnosed by using fine needle aspiration cytology, mammography, ultrasonography and incisional biopsy. Histopathological studies revealed invasive ductal carcinoma (not otherwise specified), which was of grade III in left breast and of grade II in right breast. We are presenting this case with its clinico-pathological findings, as synchronous bilateral breast cancer occurs extremely rarely in males.
Male breast cancer, Synchronous neoplasm, Bilateral
Introduction
Male breast cancer is a rare neoplasm which accounts for 1.2 – 2 % of all cancers in men and 1% of the total cases of breast cancer [1,2]. The incidence of bilateral breast cancer accounts for only 0.5 – 1 % of male breast cancers and synchronous cancers are extremely rare [3,4]. Carcinoma of the male breast has many similarities with the breast cancer which occurs in women [5]. We are reporting this case for rarity of the disease, advanced presentation of the lesion and synchronous bilateral breast cancer.
Case Report
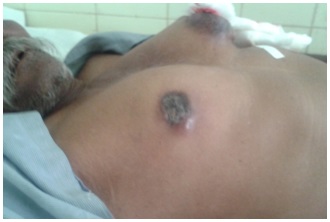
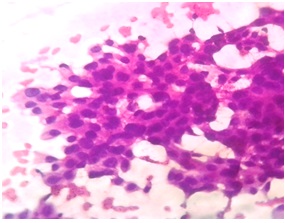
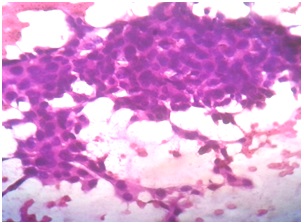
A 70-year-old male was referred to the Surgical Oncology Division of our hospital for complaint of lumps in both the breasts [Table/Fig-1] which had been seen since 4 months. Patient had first noticed the lump in left breast region, the size of which had increased rapidly, with secondary changes of ulceration, bloody discharge and oedema. One month later, he had noticed a lump in his right breast region, the size of which had increased gradually. Nipple was retracted and it showed bloody discharge. Axillary lymphadenopathy was noted on left side, on clinical examination. No lymphadenopathy was noted in right axillary region. There was no history of trauma, familial breast cancer, gynaecomastia, solid organ tumour, hormonal treatment or any other malignancies. Patient has a history of tobacco chewing since past 10 years. Patient was non alcoholic. Both his testicles were normal. The routine haematological and biochemical investigations were normal. On radiological investigations (X-rays of chest and abdomen, ultrasonography of abdomen and pelvis) no evidence of metastasis was seen. Fine Needle Aspiration Cytology (FNAC) was performed, which yielded hyper cellular smears which showed neoplastic ductal epithelial cells [Table/Fig-2,3]. The neoplastic cells were large, round to oval, having moderately pleomorphic hyperchromatic or vesicular nuclei with 1-2 prominent nucleoli and moderate amount of eosinophilic cytoplasm. Neoplastic cells were arranged in sheets, branching patterns, acini and tubules, they were scattered singly. Background of the smears showed necrotic debris, inflammatory cells, stromal fragments and haemorrhagic material. Occasionally, giant tumour cells were noted. FNAC findings were similar for both right and left breast masses. An incisional biopsy was done, which showed features of Invasive Ductal Carcinoma.
Photograph showing bilateral breast lumps showing secondary changes of ulceration, oedema and retraction of nipple
Photomicrograph of FNAC of right breast lump neoplastic cells arranged in sheets and tubular pattern (Haematoxylin Eosin stain, x 100)
Photomicrograph of FNAC of left breast lump showing large neoplastic cells having moderately pleomorphic hyperchromatic or vesicular nuclei. (Haematoxylin Eosin stain, x 100)
Bilateral toilet mastectomy with axillary node dissection was done and specimen was sent for histopathological studies. Left mastectomy specimen showed destruction of nipple areola, with an area of ulceration and a large irregular grey white, firm ulceroproliferative mass which measured 5 x 4 x 3.5 cm. Cut section of the mass was grey white, with areas of haemorrhage and necrosis. Left axillary nodes were enlarged. Right mastectomy specimen showed subareolar tumour mass which measured 4 x 3.5 x 3cm. Cut section showed an irregular, grey white tumour mass.
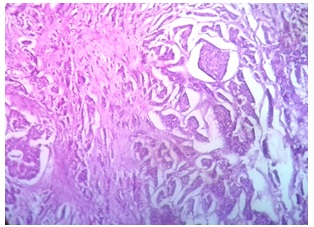
The histopathological diagnosis was given as bilateral Invasive Ductal Carcinoma (not otherwise specified)- Grade III in – Left breast and Grade II – in Right breast [Table/Fig-4], with ulceration, necrosis and tumour emboli. There was no evidence of Paget’s disease. The overlying skin showed no evidence of invasion by the tumour. Base of the resection (muscle, fascia) were free from the tumour invasion. Out of 10 left axillary lymph nodes, 2 showed evidences of metastasis. On doing immunohistochemistry, the tumour was found to be ER, PR positive and Her2/neu negative.
Photomicrograph on histopathology showing neoplastic Invasive Ductal carcinoma in glandular pattern, sheets and clusters (Haematoxylin Eosin stain, x 100)
Discussion
Bilateral male breast cancer is very rare, with a reported incidence being 0.5–1% of all male breast cancers [3,4]. Most cases of male breast cancer are detected between the ages of 60 and 70 years. The mean age is 67 years, which is older than that which is seen in women. Several risk factors have been proposed, which include familial and genetic factors radiation exposure, Klinefelter’s syndrome (47,XXY), hormonal imbalance, obesity, undescended testis, orchitis and orchectomy [6]. In our case, no such risk factor could be identified.
Clinically, most of the breast cancers in men present as lumps, bloody nipple discharges or retractions, but they are often detected late. In our case also, patient presented late with large masses, ulceration of skin, bloody nipple discharge, retraction of nipple [Table/Fig-1] and left axillary nodal involvement.
On cytology, we diagnosed our case to be positive for duct carcinoma [Table/Fig-2,3] and confirmed it by histopathology [Table/Fig-4] as Invasive Ductal Carcinoma (not otherwise specified) Grade II- in Right breast with Ductal Carcinoma In Situ (DCIS) and Grade III – in left breast, according to modified Bloom and Richardson grading system. Most of the invasive carcinomas seen in men are ductal and papillary types, while smaller portion is of lobular and mucinous type [7]. The data from Surveillance, Epidemiology and End Results (SEER) Cancer Registry has shown that among invasive carcinomas, 93.7% of male breast cancers were ductal or unclassified carcinomas, 2.6% were papillary tumors,1.8% were mucinous tumours and only 1.5% were lobular tumours [8]. This distribution is in contrast to that which is seen in female breast cancer, in which almost 12% of cancers are lobular carcinomas.
Tumour size, lymph node involvement are two clear prognostic factors for male breast cancer [8]. Tumour size is a significant prognostic marker for male breast cancer [9]. Higher survival rates of upto 85% are seen with tumour sizes of <2 cm. A higher tumour grade is associated with a poor prognosis and a significant difference in 5-year survival [10]. Men with lymph node involvement have a 50% higher risk of death than those without lymph node involvement [8].
In our case, tumour size was large, with left axillary lymph node metastasis and on doing histopathology, Grade II and Grade III invasive ductal carcinomas with secondary changes of ulceration and necrosis were seen, which favoured poor prognostic criteria.
Surgical treatment, usually mastectomy, with axillary clearance, is required. Other modality remains modified radical mastectomy. Radiotherapy, hormone therapy and chemotherapy constitute an essential part of the adjuvant therapy. In our case, a bilateral toilet mastectomy was done. Post-operative period was uneventful. Patient is on chemotherapy and he is responding well to the treatment.
Conclusion
Male breast cancer remains a rare disease, although its incidence is increasing. Synchronous, bilateral male breast cancer is an exceptional finding, as it was noted in our case. Breast cancer which occurs in males is thought to have a worse prognosis than breast cancer which occurs in women; this might be due to poor level of awareness, delayed diagnosis, increased age of onset, increased co-morbidity and a more progressive stage of disease at initial presentation.
[1]. Ravandi–Keshani F, Hayes TG, Male breast cancer: a review of literatureEur J Cancer 1998 34(9):1341-7. [Google Scholar]
[2]. Ozet A, Yavuz AA, Komurcu S, Ozturk B, Bilateral male breast cancer and prostate cancer: a case reportJPN J Clin Oncol 2000 Apr 30(4):188-90. [Google Scholar]
[3]. Kahla PB, Cassaro S, Vlandimir FG, Wayne MG, Bilateral synchronous breast cancer in a maleMt Sinai J Med 2005 72(2):120-3. [Google Scholar]
[4]. Camus MG, Joshi MG, Mackarem G, Ductal Carcinoma in situ of the male breastCancer 1994 74:1289-93. [Google Scholar]
[5]. Dos Santos VM, Cintra osterne EM, De Castro RA, Marques HV Jr, Asian Pac J CancerBilateral male breast cancer. Too many concerns? 2007 Oct 8(4):640-1. [Google Scholar]
[6]. Weiss JR, Movisch KB, Swede H, Epidemiology of male breast cancerCancer Epidemiol Biomarkers Prev 2005 14:20-6. [Google Scholar]
[7]. Stalsberg H, Thomas DB, Rosenblatt KA, Jimenez LM, Histologic types and hormone receptors in breast cancer in United States men: a population based study of 282 menCancer Causes Control 1993 4(2):143-51. [Google Scholar]
[8]. Giordano SH, Cohen DS, Buzdar AU, Breast cancer in men: a population based studyCancer 2004 101:51-7. [Google Scholar]
[9]. Yildirim E, Berberoglu U, Male breast cancer: a 22 year experienceEur J Surg Oncol 1998 24:548-52. [Google Scholar]
[10]. Ribeiro G, Swindell R, Harris M, Banerjee S, A review of the management of the male breast Carcinoma based on an analysis of 420 treated casesThe Breast 1996 5:141-6. [Google Scholar]