Comparative Evaluation of the Effect of EDTA, EDTAC, NaOCl and MTAD on Microhardness of Human Dentin – An In-vitro Study
Rama S Kalluru1, N Deepak Kumar2, Shafie Ahmed3, Emanuel Solomon Sathish4, Thumu Jayaprakash5, Roopadevi Garlapati6, Butti Sowmya7, K Narasimha Reddy8
1 Senior Lecturer, Department of Conservative Dentistry and Endodontics, Sibar Institute of Dental Sciences, Guntur, Andhra Pradesh, India.
2 Senior Lecturer, Department of Conservative Dentistry and Endodontics, RVS College of Dental Sciences, Coimbatore, Tamil Nadu, India.
3 Professor, Department of Conservative Dentistry and Endodontics, Raja Muthaiah Dental College, Chidambaram, Tamil Nadu, India.
4 Professor, Department of Conservative Dentistry and Endodontics, Raja Muthaiah Dental College, Chidambaram, Tamil Nadu, India.
5 Professor, Department of Conservative Dentistry and Endodontics, Sibar Institute of Dental Sciences, Guntur, Andhra Pradesh, India.
6 Senior Lecturer, Department of Conservative Dentistry and Endodontics, Sibar Institute of Dental Sciences, Guntur, Andhra Pradesh, India.
7 Lecturer, Department of Conservative Dentistry and Endodontics, Sibar Institute of Dental Sciences, Guntur, Andhra Pradesh, India.
8 Reader, Department of Conservative Dentistry and Endodontics, Sibar Institute of Dental Sciences, Guntur, Andhra Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rama S Kalluru, Senior Lecturer, Department of Conservative Dentistry and Endodontics, Sibar Institute of Dental Sciences, Takkellapadu, Guntur-522509, Andhra Pradesh, India.
Phone: 09959957106,
E-mail: dr.rsreddy.endo@gmail.com
Aim: The aim of this study was to evaluate the microhardness of human dentin by using four irrigating solutions.
Materials and Methodos: A total of 40 extracted mandibular premolars were selected and sectioned horizontally in the middle third of the root. Forty specimens of 4 mm thickness were embedded in acrylic resin and polished. Four test groups, each group containing ten specimens were immersed in respective irrigating solution and subjected to vicker’s microhardness test at T0, T2 and T5min.
Statistical Analysis: The data obtained were analyzed using the one way ANOVA followed by Tukey HSD method with ap=0.05 as the level for statistical significance.
Results: Suggested that there was no statistically significant difference in mean values between four experimental irrigating solutions.
Conclusion: Mixture of Tetracycline isomer i.e. Doxycycline, Citric acid and a Detergent (Tween 80) MTAD not altered the microhardness of root canal dentin significantly and seems to be an appropriate irrigating solution, because of its harmless effect on the microhardness of the root canal dentin.
Irrigating solutions, Microhardness, Root canal dentin
Introduction
Root canal instrumentation involves simultaneous action of endodontic instruments and irrigating solutions. They eliminate pre-existent organic and inorganic remnants and reduce the microbial content and its byproducts from the root canal system [1]. Successful root canal treatment is dependent on thorough cleaning of root canal system and by removing smear layer. Nygard Ostby in 1957 introduced chelating agents in endodontics for the preparation of narrow and calcified root canals. Chelation is a physico-chemical process that prompts the uptake of multivalent positive ions, reacts with the calcium ions in the Hydroxy apatite crystals and causes changes in the microstructure of the dentin and changes in Ca-P ratio (Calcium-Phosphorus ratio) of the dentin surface. Any change in the Ca-P may change the permeability and solubility characteristics of dentin [2].
Ethylene DiamineTetraacetic acid (EDTA) was the first chelator used in dentistry, capable of softening the root canal dentin, dissolving the smear layer and increasing the dentin permeability. Sodium Hypochlorite (NaOCl) has excellent antimicrobial action and capacity of dissolving organic materials. NaOCl in association with chelating agents removes the smear layer more effectively [3]. EDTAC is the combination of EDTA with a wetting agent Cetrimide. It produces clean surface on dentin walls, as well as open dentinal tubules. EDTA by combining with Cetrimide enhances its bactericidal effectiveness and improves clinical performance [4].
MTAD (Biopure) is a combination of tetracycline isomer i.e. Doxycycline, Citric acid and a Detergent (Tween 80). It has greater antimicrobial activity and has greater ability to remove the organic acid and inorganic substances from the surfaces of the roots which is facilitated by the presence of Citric acid and Tween 80 (detergent) that aids to diffuse into root canal and dentinal tubules [5]. The aim of this study was to compare and evaluate the effect of 17% EDTA, 17% EDTAC, 3% NaOCl and MTAD on microhardness of human dentin.
Materials and Methods
Forty freshly extracted human, single rooted mandibular premolar teeth with single canal were selected and stored in 10% neutral buffered formalin for two weeks under distilled water until the moment of use. The teeth were sectioned horizontally in the middle of the root with a diamond disc under water irrigation to prevent overheating. All the samples were cleaned with saline to remove the surface debris. Ten samples were embedded in each resin block to facilitate the manipulation. The specimens were ground polished with water cooled carborundum disc and the surface of the specimens was flushed with saline. Total forty samples were randomly divided into four test groups, each group containing ten specimens. Irrigating solutions were freshly prepared and buffered to a pH of approximately 7 by using pH meter and each group is immersed in respective irrigating solution. Group I – 17% EDTA, Group II - 17% EDTAC, Group III – 3% NaOCl, Group IV - MTAD. All the samples were subjected to Vicker’s microhardness test. Diamond indenter is used at 50 gm load and 15 seconds time with a speed of 1 mm/min, to determine the microhardness. In each sample three indentations were made 500 μm within the pulp-dentin interface at time intervals of T0(0min), T2 (2min) and T5(5min). The diamond shaped indentations were observed in an optical microscope with digital camera and image analysis software, allowing the accurate digital measurement of their diagonals. The average lengths of the two diagonals were used to calculate the microhardness value (MHV). The representative hardness value for each sample was obtained at the average of the results for the three indentations. At the beginning of the experiment, reference MHV’s were obtained for samples prior to immersion in the solution (t=0min). The samples were then subjected to a solution for 2 min and a second set of measurements adjacent to the previous ones was obtained. The procedure was repeated and a third set of measurements was obtained after 3 more minutes of exposure (t=5min) [Table/Fig-1a,b,candd].
Group I after exposing to 17% EDTA for 5 min,
Group II after exposing to 17% EDTAC for 5 min
Group III after exposing to 3%NaOCl for 5 min,
Group IV after exposing to MTAD for 5 min
Results
The Statistical package SPSS (Statistical Package for Social Science, Version 4) was used for statistical analysis. Mean and standard deviation were estimated from the sample for each study group. The mean values were compared by one-way Analysis of Variance (ANOVA) appropriately followed by Tukey HSD method. Tukey HSD method was employed to identify the significant groups. In the present study, the level of significance was set as p=0.05.
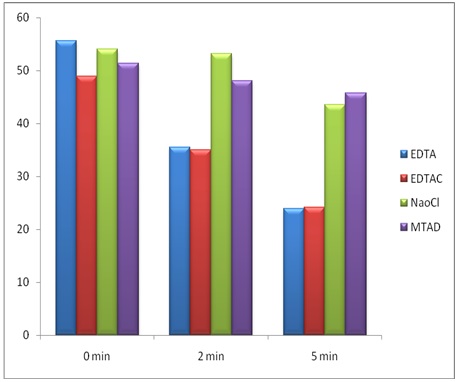
[Table/Fig-2]- Shows mean values, standard deviation between different time points for each study group. Mean values were compared in all groups and there was no statistically significant difference among the groups (p<0.001).
Mean, Standard deviation between different time points for each study group
| Groups | Time Points | n | Mean | Standard Deviation | p-value |
|---|
| 17% EDTA | 0 min | 10 | 55.5900 | 8.24250 | <0.001 |
| 2 min | 10 | 35.5000 | 2.61916 | <0.001 |
| 5 min | 10 | 23.8800 | 4.59197 | <0.001 |
| Total | 30 | 38.3233 | 14.39606 | <0.001 |
| 17% EDTAC | 0 min | 10 | 48.9300 | 7.59094 | <0.001 |
| 2 min | 10 | 35.0500 | 5.82280 | <0.001 |
| 5 min | 10 | 24.1100 | 6.79288 | <0.001 |
| Total | 30 | 36.0300 | 12.22434 | <0.001 |
| 3% NaOCl | 0 min | 10 | 54.1000 | 7.29627 | <0.001 |
| 2 min | 10 | 53.1900 | 6.81313 | <0.001 |
| 5 min | 10 | 43.5900 | 7.49584 | <0.001 |
| Total | 30 | 50.2933 | 8.47051 | <0.001 |
| MTAD | 0 min | 10 | 51.3900 | 7.04579 | <0.001 |
| 2 min | 10 | 48.0600 | 6.11486 | <0.001 |
| 5 min | 10 | 45.7800 | 6.39336 | <0.001 |
| Total | 30 | 48.4100 | 6.72204 | <0.001 |
[Table/Fig-3]- Shows the Microhardness of the relationship between different time points for each study group. At 0 mins there is no much difference in the microhardness value in all the groups. At 2 mins. NaOCl has highest microhardness value and EDTA has lowest microhardness value. At 5mins MTAD has highest microhardness value and EDTA has lowest microhardness value.
Micro hardness of the relationship between different time points for each study group
Discussion
The success of root canal therapy depends on the method and quality of cleaning, shaping, disinfection and three dimensional obturation of root canal. Cleaning with root canal irrigants is the most accepted method for removal of tissue remnants and dentin debris during instrumentation [6]. The most commonly used irrigating solutions are NaOCl, EDTA, H2O2 : Hydrogen Peroxide, Chlorhexidine and MTAD is a newly introduced irrigant. The measurement of the hardness of a material is one of the simplest non destructive mechanical characterization methods. Hardness is measured as the resistance to penetration of an indenter that is necessarily harder than the sample to be analysed [4].
The results of the present study indicate that NaOCl and MTAD do not decrease the micro hardness of root canal dentin significantly after 5 mins of exposure. Whereas EDTA and EDTAC have reduced the microhardness of root canal dentin drastically after 2 mins and 5 mins of exposure to the solutions. The decrease in microhardness is more in EDTA than EDTAC due to its chelating property. MTAD shows more microhardness than NaOCl due to its superior bactericidal activity. EDTA is the most commonly used chelating agent. The reducing effect of EDTA on dentin microhardness has been reported by Sayin et al., and they verified that EDTA alone or followed by 2.5 % NaOCl promoted a significantly greater decrease in dentin micro hardness [7].
Surfactants are used to lower the surface tension of a liquid, the interfacial tension between two liquids. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents and dispersants. Cetrimide (cetyltriethylammoniumbromide [CTAB]) is a widely used cationic surfactant. Tianyuan et al., found that surfactant additives including CTAB can somewhat elongate the hydroxyapatite nanorods, and the length- diameter ratio of hydroxyapatite nanorods decreases when the content of CTAB increases [8].
Zehender et al., reported that the effectiveness of calcium ion removal was not that effective when an endodontic solution with a wetting agent which reduces surface tension was used. This conclusion was confirmed by the present study,as EDTAC was not more effective than EDTA [9]. NaOCl is the most frequently and widely used agent for root canal irrigation.Two unique properties of NaOCl are antimicrobial activity and organic tissue dissolution. Grigoratos et al., found that NaOCl reduced modulus of elasticity and flexural strength of dentin [10]. Sluzky-Gold berg et al., demonstrated that instrumentation and irrigation with NaOCl changes the mechanical properties of dentin [3].
MTAD removes the smear layer with significantly less erosion of the dentinal tubules when compared to EDTA. MTAD has the capacity to kill E. faecalis after an exposure of 2 or 5 mins. Tetracycline has unique properties of antimicrobial efficacy, low pH, acts as calcium chelator. It causes enamel and root surface demineralization which can be comparable to that seen using citric acid. These two agents present in MTAD, but do not showed any effect on the microhardness of the dentin in the present study [5,11].
Dinesh kumar et al., in his study concluded that the microhardness of root dentin following MTAD as a final rinse was significantly less when compared to that of EDTA [12].
However, further studies are required on the effect of microhardness and the root canal irrigants in clinical situations.
Conclusion
Chelating agents EDTA, EDTAC drastically reduced the microhardness of root canal dentin, hence these agents should be used carefully. NaOCl and MTAD not altered the microhardness of root canal dentin significantly. Within the limitations of this study it is concluded that MTAD seems to be an appropriate irrigating solution, because of its harmless effect on the microhardness of root canal dentin.
[1]. Hale Ari, Ali Erdemir, Belli Sema, Evaluation of the effect of endodontic irrigation solutions on the micro hardness of root canal dentinJ Endod 2004 3:792-5. [Google Scholar]
[2]. Hulsmann M, Heckendorff M, Lennon A, Chelating agents in root canal treatment, mode of action and indications for their useInt Endod J 2003 36:810-30. [Google Scholar]
[3]. Slutzky–Goldberg I, Maree M, Liberman R, Effect of sodium hypochlorite on dentin micro hardnessJ Endod 2004 30:880-2. [Google Scholar]
[4]. De-Deus G, Paciornik S, Mauricio MHP, Evaluation of the effect of EDTA, EDTAC & Citric acid on the micro hardness of root dentineInt Endod J 2006 39:401-7. [Google Scholar]
[5]. Torabinejad M, The Antimicrobial effect of MTAD. An in vitro investigationJ Endod 2003 29:401-2. [Google Scholar]
[6]. Filho A Mcroz, Manoel D, Richardo Notak, Effect of chelating solutions on the micro hardness of human dentinJ Endod 2011 37:358-62. [Google Scholar]
[7]. Sayin TC, Serper A, Cehrelz HG, The effect of EDTA, EGTA, EDTAC and tetracycline – HCL with and without subsequent sodium hypochlorite treatment on the micro hardness of root canal dentinOral Surg Oral Med Oral Pathol Oral radiol Endod 2007 104:418-27. [Google Scholar]
[8]. Ilgan Akcay, Blge H, Sen. The effect of surfactant addition to EDTA on microhardness of root dentinJ Endod 2012 31:704-7. [Google Scholar]
[9]. Zehnder M, Reducing surface tension in endodontic chelator solutions has no effect on their ability to remove calcium from instrumented canalsJ Endod 2005 31:590-2. [Google Scholar]
[10]. Grigoratos D, Knowles J, Gulabivala K, Effect of exposing dentin to sodium hypochlorite and calcium hydroxide on its flexural strength and elastic modulusInt Endod J 2001 34:113-9. [Google Scholar]
[11]. Torabinejad M, Khademi AA, Babagoli J, A new solution for removal of smear layerJ Endod 2003 29:170-5. [Google Scholar]
[12]. Kumar Dinesh, Kumar Vinoth, Effect of ethylene diamine tetra-acetic acid, MTAD and HEBP as a final rinse on the microhardness of root dentinJ Conserve Dent 2012 15:170-2. [Google Scholar]