Rapid Immunochromatographic Test for the Identification and Discrimination of Mycobacterium tuberculosis Complex Isolates from Non-tuberculous Mycobacteria
Vishnu Prasad Shenoy1, Chiranjay Mukhopadhyay2
1 Associate Professor, Department of Microbiology, Kasturba Medical CollegeManipal, Karnataka, India.
2 Professor and Head, Department of Microbiology, Kasturba Medical CollegeManipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vishnu Prasad, Associate Professor, Department of Microbiology, Kasturba Medical College Manipal, Karnataka-576104, India.
Phone: 09886616197,
E-mail: vishnumanav@gmail.com
Background: A new rapid Immunochromatographic test (ICT) kit (SDBioline TB Ag MPT64RAPID®) developed by Standard Diagnostics, South Korea was evaluated for rapid differentiation of M. tuberculosis from non tuberculous mycobacteria (NTM). It detects MPT 64 antigen in M. tuberculosis isolates using mouse monoclonal MPT 64 antibody. The kit was assessed for routine identification of the Acid Fast Bacilli(AFB) isolated in our laboratory.
Materials and Methods: Two hundred eight culture isolates of Mycobacteria were tested using ICT test kit for detection of MPT 64 antigen from liquid and solid culture. H37Rv strain was employed as the positive reference control. Any negative result was referred for confirmation by Gen Probe Accu Probe assay for MTB Complex (Gen-Probe, San Diego, Calif.). Speciation of NTM was performed using genotypic Mycobacterium CM assay (Hain’s life sciences, Germany).
Results: Of the 208 culture positive isolates tested, 182 (87.5%) were found positive for Mycobacterium tuberculosis Complex and remaining 26 (12.5%) were considered as NTM. These results were further confirmed by Gen Probe Accu probe assay that served as the reference method for detection of MTBC. H37Rv reference strain was taken as a control for ICT test and molecular tests. The reference strain showed the presence of MPT64 antigen band in the ICT test. Similar bands were formed in all MTBC (182) isolates tested, proving 100 per cent sensitivity and no bands were detected in 48 (100%) NTM isolates tested, proving 100 per cent specificity of the ICT kit.
Conclusion: Tuberculosis is a global pandemic. Rapid identification of Mycobacteria as MTB complex or non-tuberculous Mycobacteria from culture is important for treatment of infected cases and drug susceptibility testing of the culture isolate. MPT 64 TB antigen detection using SD Bioline Immunochromatographic test is a simple and cost effective method for differentiation of Mycobacterial cultures as MTB complex from non- tuberculous Mycobacteria.
MPT 64 Antigen,, M tuberculosis complex, Accu probe, Mycobacterium CM assay
Introduction
One third of the world population is infected with Mycobacterium tuberculosis (MTB), nearly eight million new cases of tuberculosis occurs globally and two million deaths occur annually. Identifying the cases appropriately and treating these patients, especially the open cases thereby preventing the infection to contacts is crucial for successful control of the disease [1]. Conventional techniques for culture and identification of mycobacterial culture are important as the specific media not only allows the growth of MTB but also the (NTM)’s which takes a long time to grow. This in turn delays drug susceptibility tests of Mycobacterium tuberculosis by conventional method resulting in minimum of three to four months for completion of reporting after the specimens are received in the laboratory. Newer methods of culture has drastically reduced the turnaround time for culture but cannot classify the mycobacteria as MTB complex or NTM. The conventional techniques used in many of the laboratories including the reference laboratories for the identification of mycobacterial cultures are time consuming [2]. The delay in diagnosis, identification and anti-tubercular sensitivity testing of MTB complex may be one of the important factors contributing to increase in TB cases in developing countries like India [3]. Molecular tests like accu-probe assay are available for differentiation of MTB complex and NTM but require special infrastructure, trained laboratory personnel. These tests are expensive which are not advocated in resource poor settings [4].
MPT64, a 24 kDa secretory protein, is one of the major antigens from TB bacteria. MPT64 has been shown to differentiate the Mycobacterium tuberculosis complex (MTBC) from other bacterial species including the BCG strain [5,6]. Standard Diagnostics, Inc (SD) (Yongin, Korea) developed the SD BIOLINE TB AgMPT64 RAPID® test, which is a simple and rapid Immunochromatographic test using monoclonal anti-MPT64 antibody that is able to discriminate between MTBC and NTM [7,8]. The purpose of this study was to evaluate the SD Bioline Kit MPT64 Antigen detection test for routine identification of MTB complex.
Materials and Methods
A total of 2266 clinical samples, of about 2-15 ml, were collected from pulmonary sites like sputum, brochoalveolar lavage, bronchial washings and extra-pulmonary sites (cold abscesses, pleural fluid, peritoneal fluids, lymph node biopsy, tissues, urine and wound swab) from the tertiary care hospital in south coastal Karnataka, India. These samples were processed as per the institutional ethical policy for mycobacterial culture from June 2011 to May 2013.These samples were subjected to decontamination and homogenization using NaLC-NaOH method for liquid culture and modified Petroff’s method for solid culture [9,10]. Clinical samples after concentration were inoculated in liquid culture and solid culture incubated in MGIT 960 liquid culture system for six weeks and in Lowenstein Jensen (LJ) media up to eight weeks respectively. Once the culture was declared positive, preliminary smears were made and Ziehl-Neelsen’s staining was performed to confirm the isolate as AFB. A total of 208 culture isolates of Mycobacteria, isolated from liquid and solid culture, using BACTEC MGIT 960 system and Lowenstein Jensen media, were used for the study. H37Rv strain and Mycobacterium fortuitum(TMC-1529) obtained from NIRT Chennai were used as the standard positive and negative control for identification purpose.
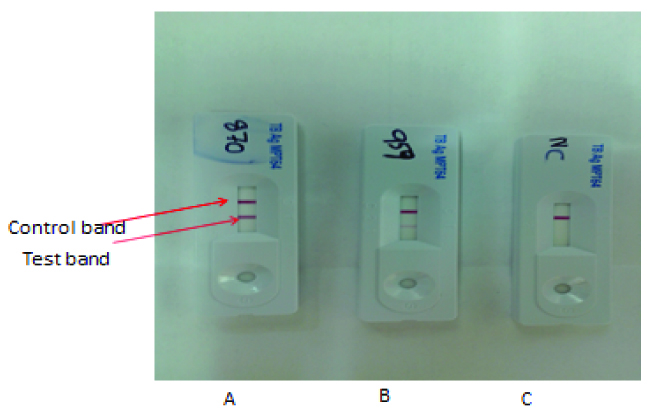
TB Antigen MPT 64 Rapid ICT kit, manufactured by Standard Diagnostics, Seoul, South Korea, was used as per the manufacturer’s instructions [11]. Monoclonal mouse antibodies, against MPT64 antigen, are immobilized on a nitrocellulose membrane for confirmation of MTB isolates. The entire test procedure was carried out inside a biosafety class II cabinet and level two biosafety procedures were followed. One hundred microlitres of a sample from liquid culture was applied in the sample well directly without any sample preparation. For solid culture isolates, 2-4 colonies were scrapped from the solid medium and suspended in 200 μL of extraction buffer provided in the kit. This extracted solution was applied in the sample well same as liquid culture. Tests were interpreted after 15 minutes of sample application at room temperature. The presence of control and test pink colored bands indicates the test is positive and presence of only the control band alone indicates negative result for MTB complex. The absence of control band after the test was considered invalid. Any negative result was referred for confirmation by Gen Probe Accu Probe assay for MTBC (Gen-Probe, San Diego, Calif). To determine the species of the NTM isolates, the GenoType Mycobacterium CM kit, a test based on the DNA-STRIP technology that permits the identification of some mycobacterial species, was used according to the manufacturer’s instructions (HainLifescience GmbH, Nehren, Germany).
Results
The SD BIOLINE TB Ag MPT64 RAPID® assay was used to test 208 strains of Mycobacteria as shown in [Table/Fig-1] and was compared with known positive and negative controls. The control band was strongly positive showing pink band in the test region for all 182 strains and H37Rv indicating the presence of MPT 64 antigen. The positive bands developed within 5-10 mins. The sensitivity and specificity of the assay was calculated based on test results from MTBC and NTM isolates. Strong positive bands were obtained for all MTBC (n = 182) and H37Rv strain regardless of the culture medium, while no positive signal was observed for any of the 26 NTM, which indicates a test sensitivity and specificity of 100% as shown in [Table/Fig-2]. Those strains which showed faint band or negative result [Table/Fig-3] were further subjected to Accuprobe assay for confirmation of MTBC and those which were identified as NTM by probe assay were speciated using Genotype Mycobacterium CM Kit [Table/Fig-1].
Different Mycobacterial Isolates Tested With Sd Bioline Tb Ag MPT64 Rapid Test
| Species | Isolates |
|---|
| H37Rv (Standard strain) | 1 |
| Mycobacterium tuberculosis complex | 182 |
| Non Tuberculous Mycobacteria | 26 |
| Mycobacterium fortuitum (TMC-1529)(negative control) | 1 |
| Mycobacterium fortuitum | 1 |
| Mycobacterium kansasii | 1 |
| Mycobacterium scrofulaceum | 1 |
| Mycobacterium chelonae | 1 |
| Mycobacterium intercellulare | 4 |
| Mycobacterium abscessus | 9 |
| NTM unidentified | 9 |
Sensitivity and Specificity of SD Bioline TB Ag MPT64 Rapid test
| Culture method | Liquid culture | Solid culture |
|---|
| Test method | | MTBC | NTM | MTBC | NTM |
|---|
| SD Bioline MPT 64 Rapid method | Positive | 147 | 0 | 35 | 0 |
| Negative | 0 | 21 | 0 | 5 |
| Total | 208 |
| Sensitivity & Specificity (%) | 100 |
MTBC – Mycobacterium tuberculosis Complex
NTM - Non Tuberculous Mycobacteria
Identification of Mycobacterium tuberculosis Complex by MPT64 ICT Kit
Discussion
Tuberculosis is a global pandemic and a major public health problem in a country like India. The diagnostic tools available for identification and discrimination of MTBC from NTM have low sensitivity. Smear for AFB is rapid but the sensitivity has not been evaluated and cannot discriminate between MTBC and NTM. There is an urgent need for a simple, sensitive and accurate test for the differentiation of Mycobacteria. Modern techniques like nucleic acid amplification, chemiluminescent probes, HPLC and 16S rRNA genes are more technical demanding methods and are expensive [12–14]. It was therefore decided to test the utility of “SD BIOLINE TB Ag MPT 64®” assay marketed as a rapid test and can be used in simple laboratory settings. This study has evaluated the performance of the ICT for the identification of MTB complex from positive culture samples. The result presented, indicates that SD bioline TB Ag identification had 100% sensitivity and specificity for the strains, compared with the conventional methods of identification which present an excellent agreement. These results are similar to those observed by other studies [15,16]. Several cases of MTBC have been reported as negative test results. Hirano et al suggested a possible explanation that in these tests-strains had mutated within the mpt64 gene which would have led to the production of incomplete protein [17]. False negativity may be also due to the low expression of the MPT 64 antigen by some isolates [18]. In these cases more specific techniques for the diagnosis of MTB should be implemented if clinical symptoms and microbiological evidence are suggestive for Tuberculosis. No positive bands were observed for any NTM bacilli in this study as reported in previous studies [5]. Few authors although have reported false positive results when evaluating for MPT64 antigen [5,17,19]. Usage of SD MPT64 antigen has not reported any false positive among the NTM species as reported by Toihir et al., [20].
Even though doubtful results were sometimes observed in this study due to faint bands produced by MTBC, there were no discrepancies in the interpretation of SD Bioline MPT64 Rapid Antigen test as all of them were confirmed positive by accuprobe assay. However it is important to identify the Mycobacterium which was shown negative by MPT 64 Antigen test, to be excluded from the MTBC group, which is important for effective treatment of clinical cases and for performing DST in the laboratory. This test is applicable for both liquid and solid culture specimens, which requires the manipulation of bacterial culture, thus it is compulsory to use biosafety cabinet level II to ensure laboratory safety.
The test is simple and easy to perform; it does not require any special instrumentation or sample preparation. SD Bioline MPT64 Rapid Antigen detection test is rapid as the time required for the complete assay is less than 30 minutes compared to few hours or days required for molecular characterization and phenotypic tests. SD Bioline MPT64 Rapid Antigen detection test is not very costly and is available in a pack size of 25 cassettes which are individually packed. This helps the laboratory technologists only to use the required test cassettes without any kit wastages. Thus this kit suits for the use of any basic laboratory performing mycobacterial culture and can replace conventional biochemical tests which take long time for identification with additional limitations of using positive and negative controls for each lot of phenotypic tests [21]. Molecular tests on the contrary requires special set up and costs more than 1000.00 per isolate identification and are not able to identify nor differentiate the members of MTB complex [7].
Conclusion
This study has demonstrated the importance of using this test for the clinical identification of MTB complex. However when the test results are negative, alternative specific identification tests can be performed for the confirmation of MTBC and NTM isolates. No false positivity was observed with the routinely isolated NTM in our laboratory. Thus SD Bioline MPT64 Rapid Antigen detection test appears reliable and has a great potential to replace phenotypic characterization which will rapidly facilitate decisions in the management of tuberculosis.
MTBC – Mycobacterium tuberculosis Complex
NTM - Non Tuberculous Mycobacteria
[1]. Kunnath-Velayudhan S, Gennaro ML, Immunodiagnosis of tuberculosis: a dynamic view of biomarker discoveryClin Microbiol Rev 2011 24:792-805. [Google Scholar]
[2]. Wang JY, Lee LN, Hsu HL, Hsueh PR, Luh KT, Performance assessment of the DR. MTBC Screen assay and the BD ProbeTec ET system for direct detection of Mycobacterium tuberculosis in respiratory specimensJ ClinMicrobiol 2006 44:716-9. [Google Scholar]
[3]. Nair D, Capoor MR, Rawat D, Srivastava L, Aggarwal P, Standardization of first and second-line antitubercular susceptibility testing using BacT Alert 3D system: a report from a tertiary care centre in IndiaBraz J Infect Dis 2009 13:422-26. [Google Scholar]
[4]. Johansen IS, Rapid diagnosis of mycobacterial diseases, and their implications on clinical managementDan. Med. Bull 2006 53:28-45. [Google Scholar]
[5]. Abe C, Hirano K, Tomiyama T, Simple and rapid identification of the Mycobacterium tuberculosis complex by immunochromatographic assay using anti-MPB64 monoclonal antibodiesJ. Clin. Microbiol 1999 37:3693-7. [Google Scholar]
[6]. Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I, Proteins released from Mycobacterium tuberculosis during growthInfect Immun 1991 59:1905-10. [Google Scholar]
[7]. Kanade S, Nataraj G, Suryawanshi R, Mehta P, Utility of MPT 64 antigen detection assay for rapid characterization of mycobacteria in a resource constrained settingIndian J Tuberc 2012 59:92-6. [Google Scholar]
[8]. Maurya AK, Nag VL, Kant S, Kushwaha RAS, Kumar M, Mishra V, Evaluation of an immunochromatographic test for discrimination between Mycobacterium tuberculosis complex & non tuberculous mycobacteria in clinical isolates from extra-pulmonary tuberculosisIndian J Med Res 2012 135:901-6. [Google Scholar]
[9]. Scott CP, Dos AnjosFilho L, De Queiroz Mello FC, Thornton CG, Bishai WR, Fonseca LS, Comparison of C(18)-carboxypropylbetaine and standard N-acetyl-L-cysteine-NaOH processing of respiratory specimens for increasing tuberculosis smear sensitivity in BrazilJ ClinMicrobiol 2002 40:3219-22. [Google Scholar]
[10]. WHO, Laboratory Services in Tuberculosis Control. Part III. Culture, WHO, Geneva, Switzerland, 1998 [Google Scholar]
[11]. TB Antigen MPT 64 Rapid. Available from http://www.standardia.com/html_e/mn03/mn03_01_00.asp?intId=99 [Google Scholar]
[12]. French AL, Benator DA, Gordin FM, Nontuberculous mycobacterial infectionsMed Clin North Am 1997 81:361-79. [Google Scholar]
[13]. Ichiyama S, Iinuma Y, Yamori S, Hasegawa Y, Shimokata K, Nakashima N, Mycobacterium growth indicator tube testing in conjunction with the AccuProbe or the AMPLICOR-PCR assay for detecting and identifying mycobacteria from sputum samplesJ ClinMicrobiol 1997 35:2022-5. [Google Scholar]
[14]. Laughon BE, New tuberculosis drugs in developmentCurr Top Med Chem 2007 7:463-73. [Google Scholar]
[15]. Marzouk M, Kahla IB, Hannachi N, Ferjeni A, Salma WB, Ghezal S, Evaluation of an immunochromatographic assay for rapid identification of Mycobacterium tuberculosis complex in clinical isolatesDiagn Microbiol Infect Dis 2011 69:396-9. [Google Scholar]
[16]. Martin A, Bombeeck D, Mulders W, Fissette K, DeRijk P, Palomino JC, Evaluation of the TB Ag MPT64 Rapid test for the identification of Mycobacterium tuberculosis complexInt J TubercLung Dis 2011 15(5):703-5. [Google Scholar]
[17]. Hirano K, Aono A, Takahashi M, Abe C, Mutations including IS6110 insertion in the gene encoding the MPB64 protein of Capilia TB negative Mycobacterium tuberculosis isolatesJ ClinMicrobiol 2004 42:390-2. [Google Scholar]
[18]. Park MY, Kim YJ, Hwang SH, Kim HH, Lee EY, Jeong SH, Evaluation of an immunochromatographic assay kit for rapid identification of Mycobacterium tuberculosis complex in clinical isolatesJ ClinMicrobiol 2009 47:481-4. [Google Scholar]
[19]. Gaillard T, Fabre M, Martinaud C, Vong R, Brisou P, Soler C, Assessment of the SD Bioline Ag MPT64 Rapid ” and the MGIT” TBc identification tests for the diagnosis of tuberculosisDiagnMicrobiol Infect Dis 2011 70:154-6. [Google Scholar]
[20]. Toihir AH, Rasolofo V, Andrianarisoa SH, Ranjalahy GM, Ramarokoto H, Validation of an immunochromatographic assay kit for the identification of the Mycobacterium tuberculosis complexMem Inst Oswaldo Cruz 2011 Sep 106(6):777-80. [Google Scholar]
[21]. Kumar VG, Urs TA, Ranganath RR, MPT 64 antigen detection for rapid confirmation of M. tuberculosis isolatesBMC Res Notes 2011 4:79 [Google Scholar]