Of the metabolic syndromes, Diabetes Mellitus (DM) is prevalent worldwide among all age groups. India has the world’s largest diabetic population, with an estimated 50.8 million people living with DM. World Diabetes Foundation stated that 50 per cent of the 285 million adult population afflicted with DM are women. Two out of every five women afflicted fall in the reproductive age group.
The relative importance of impaired insulin release and insulin resistance in the pathogenesis of Type 2 diabetes had been evaluated by several studies, but understanding of the exact pathogenesis had been complicated by several factors [1–3]. Few authors have reported that hyperglycemia stimulates the release of the inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) from cells such as monocytes [4–8], secretion of acute phase reactants by adipocytes and CRP by liver cells [9]. Among several markers of inflammation like adiponectin and nuclear factor kappa-light chain enhancer of activated B cells(NF-kB), only hs–CRP level showed a positive correlation with fasting and post prandial glucose level consistently [10]. Another prospective evaluation study proved hs-CRP as the strongest and most significant of the 12 inflammatory markers in women that predicted risk for future cardiovascular events [11].
The strong relationship between hs-CRP and Type 2 DM was proved by some cross-sectional studies irrespective of ethnicity [9–22] and recommended using adapted normal reference range for menstruating women during follicular phase [14]. Among them, the results of few studies showed more pronounced increase in CRP concentration associated with risk factor clustering, across the spectrum of fasting glucose level in women than men [7,15,16,17,19]. Data analysis from two population-based studies showed elevated hs-CRP levels associated even with prediabetic state [23].
Past studies found similar levels of insulin sensitivity and glucose tolerance during all the three phases of the menstrual cycle in normal women [24, 25, 26]. But few contrasting studies showed hs-CRP to be elevated/ depressed by progesterone/estrogen hormones with respect to different phases of menstrual cycle [27] and impaired glucose metabolism, depressed insulin sensitivity in the luteal phase of menstrual cycle [28–30] and stressed the need for controlling steroid hormone levels during comparison of CRP and inflammation across population. Increase in level of inflammatory mediators in menopausal women paralleled decrease in sex steroid level with a rise in cardiovascular risk [31, 32, 33]. A cross-sectional study recorded unsustained menstrual cycle-dependent changes in systemic IL-6 plasma levels, differentially regulated changes in α1-acid glycoprotein (AGP) level, and increase in CRP levels with increase in progesterone levels [33].
Only few studies exist regarding hs-CRP levels in Asian Indians (a high risk group for occurrence of Type 2 diabetes) [34–38]. Moreover, to our knowledge, no studies as on date have comprehensively assessed the relationship between hs-CRP, reproductive hormones and glycemic levels of Type 2 diabetics in Asian Indian women . And so, we designed this study to fill the lacunae in our knowledge by way of testing the relationship between varying levels of hs-CRP in different phases of menstrual cycle to good /poor glycemic control in Type 2 diabetic women of reproductive age group with respect to reproductive hormones.
To assess the variability in glycemic control during different phases of menstrual cycle in Type 2 diabetic women using high sensitivity C- reactive protein levels.
Materials and Methods
Method of selection of Participants
We carried out a Prospective study of Evaluative research design in the institution’s Diabetic outpatient clinic. We enrolled 50 Premenopausal Type 2 diabetic women in the age group of 19–50 years, who satisfied the inclusion and exclusion criteria for the study.
We included women with regular 26–33 day menstrual cycle, established or newly diagnosed Type 2 DM without any diabetes related complications or hypertension and those who were on oral anti-diabetic drug therapy irrespective of their body mass index in this study.
Women having menstrual irregularities, acute infections (within a month prior to study period), established inflammatory diseases (acute and chronic), who were on exogenous hormones, hormonal contraceptives, intrauterine devices (within last six months) and who were also infertile, smokers, alcoholics were excluded from our study. Women who were on thiazolidinedione group of anti-diabetic drugs were also excluded from the study [39].
Institute Ethical committee had approved the proposal and the Helsinki guidelines for ethical human experimentation were followed. A maximum of five patients per day were interviewed during the study period. Enrolled subjects details were recorded in the case study form. We advised the enrolled patients to avoid any drastic changes in their daily routine work, diet, exercise and the drug regimen during the study period. We also instructed them to report any minor/major illness and any other type of drug intake during the study period.
Identification of Ovulation
We used basal body temperature technique for identification of ovulation, contrary to the intermittent sampling method used by a previous study [40]. We trained the participants to check the time of ovulation by the basal body temperature technique, one cycle prior to the study menstrual cycle. During study menstrual cycle period, they reported early in the morning on the 2nd day after the menstrual bleeding stops and then again three days after ovulation.
Assays
We used venipuncture method for collecting blood for assay, in comparison to dried blood spot(DBS) assay as used by previous researchers [41]. So, with sterile aseptic and antiseptic technique we took 5 ml of fasting intravenous blood with disposable syringe. The blood samples collected were processed for fasting blood sugar values by glucose oxidase – peroxidase (GOD-POD) method in fully automated analyser. In concurrence with previous studies, we analyzed the reproductive hormones using ELISA KIT for only nine samples [14, 27], from among the 42 responders. The hs-CRP was measured using latex enhanced turbidimetric immunoassay.
The whole experiment and its results were maintained with confidentiality.
Statistical Analysis
We analysed the observed values using Pearson’s correlation (2-tailed) and significance test to know significant correlation between fasting blood sugar values and hs-CRP in follicular and luteal phase and hs-CRP and endogenous sex hormones. All results were interpreted as statistically significant at p value < 0.05.
Observations and Results
We encountered eight non-responders, out of the 50 diabetic women enrolled for the study. And so, we studied only 42 women between19-50 years. Their mean age was 38 ±6.49 years. The mean cycle length was 29±2 days. We observed abnormally elevated hs-CRP level in 8 participants and so we excluded them from statistical analysis, as such an elevation could be only due to a possible systemic infection.
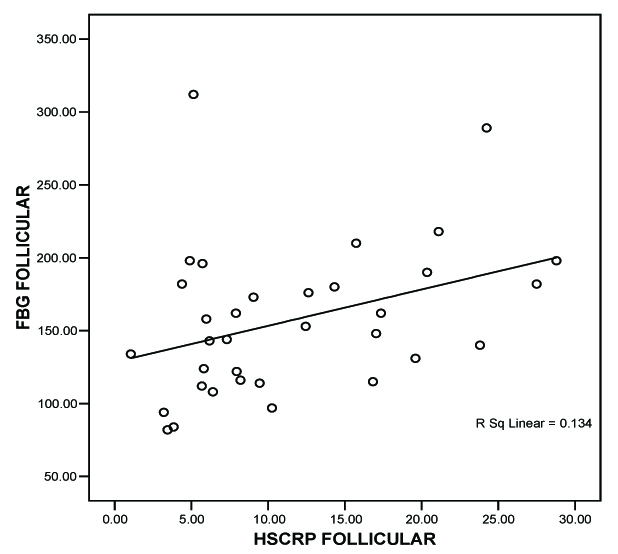
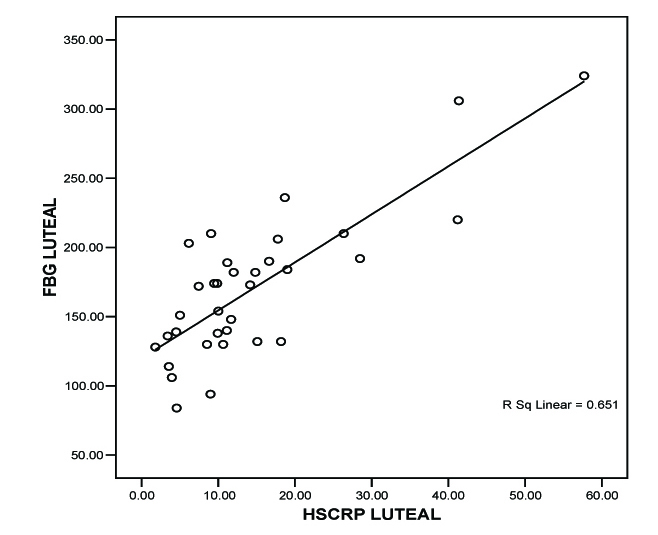
In the follicular phase, the range of fasting blood sugar was 82-312mg/dl with a mean of 157.2647 mg/dl (n=34). In the luteal phase, fasting blood sugar had a range of 84–324 mg/dl with mean of 170.0882 mg/dl (n=34). Even when analyzed separately in each subject, the hs-CRP values were low in the follicular phase when compared to the luteal phase of menstrual cycle. The mean hs-CRP in the follicular and luteal phase was 11.5771 mg/l and 14.4812 mg/l respectively [Table/Fig-1].
Fasting Blood glucose(FBG) ,Hs-CRP ,Sex steroid levels in Follicular and Luteal phase
| N | Minimum | Maximum | Mean | Std. Deviation |
|---|
| FBG (mg/dl)–Follicular phase | 34 | 82.00 | 312.00 | 157.2647 | 51.90569 |
| FBG (mg/dl )–Luteal phase | 34 | 84.00 | 324.00 | 170.0882 | 52.17242 |
| Hs-CRP (mg/l)-Follicular phase | 34 | 1.05 | 28.79 | 11.5771 | 7.61389 |
| Hs-CRP (mg/l)-Luteal phase | 34 | 1.80 | 57.68 | 14.4812 | 12.13440 |
| Estrodial (pg/ml)-Follicular phase | 9 | 52.50 | 225.80 | 146.9111 | 58.41627 |
| Progesterone (ng/ml)- Luteal phase | 9 | .39 | 18.13 | 6.6444 | 6.63892 |
| Valid N (list wise) | 0 | | | | |
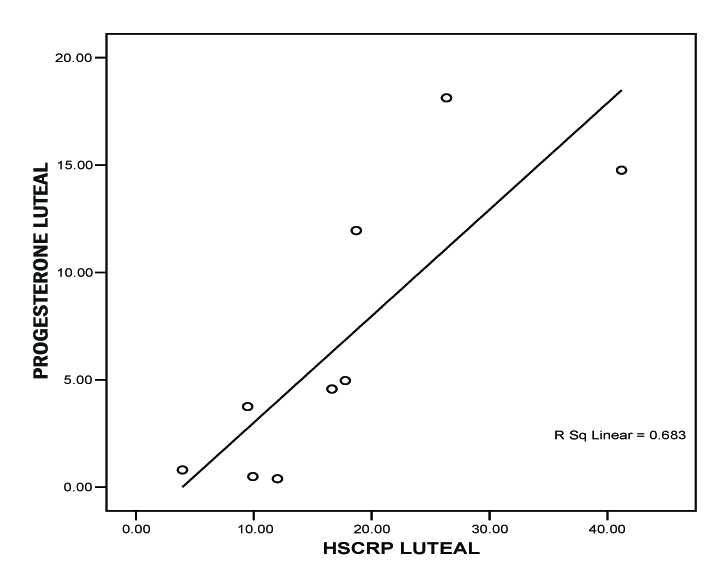
We correlated fasting blood glucose (FBG) level with hs-CRP level during both phases of menstrual cycle by Pearson correlation test. We correlated endogenous sex hormones with the respective hs-CRP levels. We concluded a positive correlation between hs- CRP and FBG during follicular phase by the value of r = 0.365 *(* Correlation is significant at the 0.05 level (2-tailed), [Table/Fig-2]. we also found a statistically significant correlation between hs-CRP and FBG during luteal phase by the value r = 0.807* * (**Correlation is significant at the 0.01 level (2-tailed), [Table/Fig-3]. We concluded from the correlation value of r = –0.311 that there is statistically insignificant correlation between Estradiol and hs-CRP. We got a positive correlation between progesterone and hs-CRP with r = 0.826** (**Correlation is significant at the 0.01 level (2-tailed), [Table/Fig-4].
FBG (mg/dl) vs hs-CRP (mg/l)-Follicular phase
FBG (mg/dl) vs hs-CRP (mg/l)-Luteal phase
Progesterone (ng/ml) vs hs-CRP (mg/l)-Luteal phase
Discussion
The purpose of this evaluative study was to determine variability in glycemic control during different phases of menstrual cycle in type 2 diabetic patients using hs-CRP as the bio marker.
Most of the studies on relationship between hs-CRP and diabetics were large population based case or case-control studies done in western, European and south Asian population. But the present study was conducted in the south Indians, the very high risk group for developing diabetes mellitus [35–37].
In previous studies, the mean age of the Type 2 diabetic subjects were 46.54 years, 54 years, 66±9 years respectively [17, 19, 20]. But the mean age of 38years in the present study points that early incidence of diabetes occurs in the high risk Indian population.
The level of hs-CRP in follicular (1.05–28.79) and in luteal phases (1.80–57.68) showed similar range and mean as the past studies [7, 13]. But few authors observed low range of hs-CRP concentration [9, 20] in diabetic patients. Studies that compared hs-CRP level between European and south Asian population and between diabetic and non diabetic subjects, found two fold and three fold rises in level of hs-CRP respectively [21, 38]. Analysis of the above studies proves that South Indian’s have higher baseline range of hs-CRP, compared to other groups of population.
Inflammatory processes may be of particular importance in the pathogenesis of Type 2 DM in women [7, 18]. Increase in hs-CRP level continuously across the spectrum of fasting blood glucose in the present study, validated the above statement. It is consistent with most of the previous studies by multiple authors except one who found hs-crp not associated with fasting blood glucose level [9, 10, 11, 15, 16, 17,18,19, 20]. Also, the association was stronger in women and diabetic subjects after multivariate adjustments in all these previous studies [7, 13, 15, 16, 17, 18, 19, 23, 38].
Impaired glucose metabolism during luteal phases of menstrual cycle and/or decreased insulin sensitivity during luteal phase may be the reason for the observed high fasting blood glucose level in the luteal phase of menstrual cycle, compared to the follicular phase in Type 2 diabetic premenstrual women in the present study. The finding was consistent with conclusion of many previous studies [13, 26, 28, 29, 30], except the study by few authors who found no variability in measures of insulin sensitivity and glucose mediated metabolism within and between menstrual phases [24, 25, 27].
In premenopausal women, the menstrual cycle represents a continuous state of change in terms of the female sex steroid environment. A strong negative relation between CRP concentration and endogenous estrogen in follicular phase and positive relation with endogenous progesterone in luteal phase and CRP levels increase with increase in progesterone levels in the present study coincides with findings of some previous studies [14, 27, 30]. This finding can be attributed to an over-all inflammatory effect of progesterone which exhibits chemotactic activity of neutrophils and increases production of inflammatory mediators.
Conclusion
The finding of the early incidence of the disease in this study, stresses the fact that diabetes is no longer an old age disease. The result shows that hs-CRP level significantly correlates with increasing levels of fasting blood glucose level in both the phases of menstrual cycle in Type 2 diabetic women. But the significance is statistically stronger only during luteal phase of the cycle (p = 0.05).
Estrodiol and hs-CRP levels showed insignificant correlation. But hs-CRP level increased significantly during luteal phase. This study concludes that Type 2 diabetic women of reproductive age group encounters a poor glycemic control during luteal phase mediated by endogenous progesterone hormone. And so, these women need specific adjustments in physical activity, titration of the anti-diabetic drug regimen and separate normal reference range for hormonal assays during the luteal phase of menstrual cycle.
A famous analogy states “Diabetes control is like riding a rollercoaster. But a woman with diabetes gets to ride an additional rollercoaster at the same time; the extra ups and downs are caused by her menstrual cycle. The goal is to turn the rollercoaster into a go cart running on a flat track by eliminating the extreme ups and downs in glycemic level.” We also conclude that the main goal of management in Type 2 diabetic women is to eliminate fluctuations in the glycemic control during the different phases of menstrual cycle so as to prevent cardiovascular and other complications associated with diabetes mellitus.
Suggestions
We recommend future investigations that probe the role of life style modifications and associated decrease in risk for cardiovascular disease occurrence in a large sample of Indian type2 diabetic women of reproductive age group.