Geographical Patterns in Antimicrobial Resistance of Acinetobacter in Clinical Isolates
Naz Perween1, Sonal Sehgal2, S. Krishna Prakash3
1 Senior Resident, Department of Microbiology, Maulana Azad Medical College, New Delhi, India.
2 Post Graduate Student,Department of Microbiology, Maulana Azad Medical College, New Delhi, India.
3 Director Professor, Department of Microbiology, Maulana Azad Medical College, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Naz Perween, Senior Resident, Department of Microbiology, Pathology Block, Maulana Azad Medical College Bahadur Shah Zafar Marg, New Delhi-110002, India.
Phone: +91-9868112294,
E-mail: drnaz07@gmail.com
Objectives:Acinetobacter spp. has emerged as a threat to the healthcare workers throughout the globe, owing to its property of multidrug resistance. The aim of the present study was to evaluate the antimicrobial resistance patterns of Acinetobacter spp. among indoor and out patients in our hospital and compare the resistance patterns in India and abroad.
Materials and Methods: In this retrospective study, which was carried out over a period of one year, a total of 5593 clinical specimens of pus and purulent fluids were examined and antimicrobial resistance pattern for Acinetobacter spp. using Modified Stoke’s were evaluated. Also a comparison was done with the other similar studies.
Statistical Analysis: Using the proportions of sensitive and resistant, the statistical analysis was done. The total, mean and percentage were calculated by using SPSS.
Results: A high level of antimicrobial multidrug-resistance was found in almost all the clinical isolate. Our study was also found to be concordant with the results of other studies.
Conclusion: There is an emerging need for identification of the genes and mechanisms for multidrug resistance among Acinetobacter spp.
Nosocomial infections, Acinetobacter spp., Multidrug-Resistance
Introduction
Acinetobacter, a ubiquitous gram negative coccobacillus was considered to be a quiet spectator till its role in nosocomial infection was described [1,2]. Now it has been proven time and again that Acinetobacter spp. have emerged globally as a challenging pathogen in causing healthcare associated infections. Acinetobacter baumannii Complex has emerged as a major culprit in the recent years. The striking ability of the Acinetobacter spp. in developing fast growing resistance to various drugs has led it to cause a variety of multi-drug resistant infections including Ventilator Associated Pneumonia (VAP), skin and soft tissue infections, urinary tract infections, meningitis, septicaemia etc [3–5].
Literature cites term, such as Multiple Drug Resistant (MDR) and Pan Drug Resistant (PDR) [6], to characterize isolates of Acinetobacter baumannii. Although no fixed definitions have been given for these terms, MDR or PDR are A.baumannii isolates that are resistant to all commercially available antimicrobial except Colistin [7,8].
Acinetobacter, the major species of A.baumannii, are very difficult to treat and combination therapy is required for effective treatment [9,10]. A wide array of antimicrobial resistance pattern have been described for Acinetobacter spp., the ones which top the list are production of beta lactamases, alteration in membrane permeability, alterations in penicillin binding protein and presence of various efflux pumps. Of these carbapenem hydrolysing Beta-lactamases which can be either metallo-Beta-lactamase (MBLs) or serine carbapenemases play a major role in multidrug resistance [3].
The functional classification of Beta lactamases as described by Bush and Jacoby [11]. are represented into four major groups 1,2,3 and 4, and subgroups a to f. The molecular classification described by Ambler [12] classifies Beta lactamases into four classes A, B,C and D. Out of these class A has not been described for A.baumannii and class B is of utmost importance in Indian scenario. According to these two classifications, ESBL belongs to class A and 2be subgroup. Carbapenemase enzyme family belongs to 2df and class D. Most predominant MBL enzyme family belongs to 3a subgroups and class B. Class D or oxacillinases have been characterized from A.baumannii and include bla oxa genes.
Aims and Objectives
A retrospective study was conducted in Department of microbiology, Maulana Azad Medical College, New Delhi,India for a period of one year, in which Acinetobacter baumannii antimicrobial resistance pattern was observed and studied from various clinical isolates. Also comparison was done to study the variations in trend in the antimicrobial resistances of Acinetobacter baumannii isolates from clinical isolates of Patients from various tertiary care hospitals in Delhi and other parts of India and abroad. The study is cleared by the ethical committee of the Maulana Azad Medical College.
Materials and Methods
Clinical specimens of pus and purulent fluids received in the microbiology laboratory of Maulana Azad Medical College over a one year period (Jan-Dec 2013) were considered the specimens under this study. The specimens were inoculated onto 5% Sheep blood agar, MacConkey agar and Brain-heart infusion broth and were observed for growth after overnight incubation at 37°C. Plates that did not show any growth were discarded the next day. Broths that did not show any growth were evaluated for turbidity for a maximum of 48hrs. Turbid broths were sub-cultured onto 5% Sheep blood agar and MacConkey agar.
Colonies obtained on solid culture media were further subjected to standard biochemical tests. Only isolates identified as Acinetobacter baumannii were selected for this study.
All isolates of Acinetobacter baumannii so obtained were subjected to antimicrobial susceptibility testing against a panel of antibiotics and their resistance patterns recorded. The following antibiotics were tested: Ciprofloxacin (5 mcg), Amoxicillin (20mcg), Gentamicin (10mcg), Ceftriaxone, (30mcg) Ceftazidime (30mcg), Cefotaxime (30mcg), Amikacin (30mcg), Imipenem (10mcg), Meropenem (10mcg) Colistin (10mcg), Polymyxin B (300U), Piperacillin-Tazobactam (100/10mcg), Netilmicin (30mcg).
The results of antimicrobial resistance were carried out using Modified Stokes method. The bacteria was cultured on nutrient agar and the antibiotic discs were used for finding out pattern of resistance using disc diffusion method [Table/Fig-1].
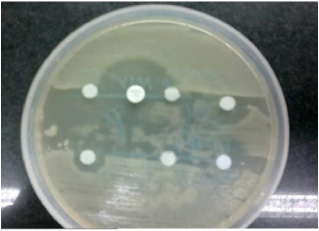
Results
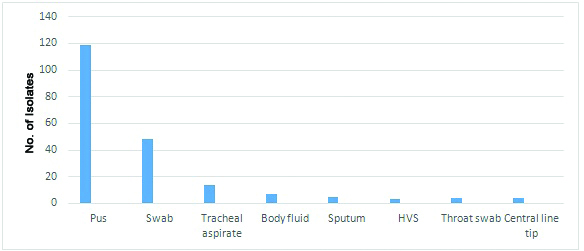
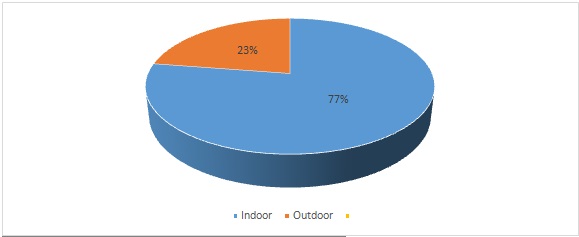
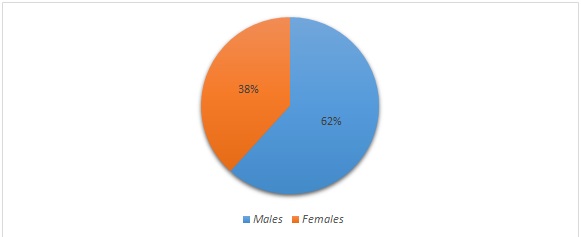
Out of a total of 5593 clinical specimens of pus and purulent fluids received in the laboratory during the study period, a total of 205(3.64%) isolates of Acinetobacter baumannii were obtained. The source of these isolates was : Pus (119), swabs from various wound sites(48), tracheal aspirates(15), body fluids(07), sputum(05),(HVS) High vaginal swabs(03), throat swab(04) and central line tip(04) shown in [Table/Fig-2]. Of all the 205 samples 158 isolates were from the patient in indoor department and rest 47 were from Outpatient department [Table/Fig-3]. The clinical isolates isolating Acinetobacter had a sex distribution of males 61.76% contributing to 127 samples and females 38.23% contributing to 78 samples [Table/Fig-4].
Proportion of outdoor and indoor patients
The antimicrobial resistance pattern in our study identified many isolates which showed the presence of multidrug resistant Acinetobacter spp. a high number of isolates were resistant to Cephalosporins like Ceftriaxone, Cephalexin, Imipenem and Meropenem resistant strains were also quite a few in number. Although Colistin had a good antimicrobial sensitivity [Table/Fig-5].
Antimicrobial Susceptibility Patterns
| Antimicrobial | % Resistance | % Sensitive |
|---|
| Ciprofloxacin | 75.64 | 24.36 |
| Amoxicillin | 88.60 | 10.46 |
| Gentamicin | 82.72 | 17.27 |
| Ceftriaxone | 79.89 | 20.12 |
| Ceftazidime | 89.44 | 10.56 |
| Cefotaxime | 80.54 | 19.45 |
| Amikacin | 88.95 | 11.03 |
| Imipenem | 15.21 | 84.77 |
| Meropenem | 7.32 | 92.68 |
| Colistin | 1.36 | 68.62 |
| Polymyxin B | 1.49 | 98.51 |
| Piperacillin-Tazobactam | 32.97 | 67.01 |
| Netilmicin | 39.02 | 85.35 |
Discussion
Over the past decade multidrug resistant Acinetobacter baumannii complex has emerged as a serious nosocomial pathogen throughout the globe.The healthcare and economic impact of A.baumannii infections, particularly in intensive care units, highlight the need to develop new approaches to treat and prevent such infections. Antimicrobial susceptibility pattern in our study showed high resistance to antibiotics like Ceftazidime (89.44%), Amikacin (88.95%), Amoxicillin (88.60%), Cefotaxime (80.54%), Gentamycin (82.72%). Acinetobacter spp. were moderately resistant to Piperacillin-Tazobactam (32.97%) and Netilmycin (39.02%). On the other hand low resistance pattern were found for Imepenem (15.21%) Meropenem (7.32%), Polymyxin B (1.49%) and Colistin (1.36%). Thus, Acinetobacter baumannii strains were highly susceptible to Carbapenems, Polymyxin B and mostly with Colistin. Other studies have reported that 12% Acinetobacter spp. Showed resistance to the standard Antibiotics, 80% to Aminoglycosides and Ciprofloxacin, 70% to Ceftazidime, 20-30% to beta lactam and Beta lactam inhibitor [3,13].
In a study conducted by Japoni –Nejad A et al., [14] in Iran in 2012, it was found out that all clinical isolates which cultured acinetobacter were resistant to Cefoxitin(56%),Cefotaxime(56%),Ceftazidime(56%),Cefopime(56%). Piperacillin-Tazobactam(56%), Aztreonam (56%), Ciprofloxacin (56%), Erythromycin(56%) and contrary to our study a high number of isolated were resistant to Meropenem (84%), Imipenem(84%) and Amikacin 80%, although Colistin had a good antimicrobial activity. Pan drug resistant strain were found out to be 11% of the total. These results were found to be similar to our study with resistance to ciprofloxacin 75.64%, Amoxicillin 88.60%,Cefotaxime 80.54%, Amikacin 88.95%.While Carbapenems like Meropenem 7.32%and Imipenem 15.21 %were found to have lesser resistance as compared to the comparative study with both Meropenem and Imipenem showing resistance of 84%. Irfan et al., conducted a study in Karachi which shows that 96.6% of the Carbapenem resistance was present in Acinetobacter baumannii [15].
Luzzaro et al., from Italy had reported 90% of isolates from their hospital to be resistant to first line drugs, with Imipenem resistance being 50% [16].
In one more study conducted in AIIMS in 2010, New Delhi, India resistance to Imipenem was found in 17.32% isolates. In another study conducted by Anitha et al., [3] in Vellore 96.5% resistance to Carbapenems was reported. The results of these studies are in concordance to our study with 15.21% resistance to Imipenem. Mumbai [3] has published a report which showed that 29% Acinetobacter isolates were resistant to Imipenem, which is again in concordance to our study. The table below shows a comparison between the susceptibility patterns between our study and studies conducted in various parts of India [Table/Fig-6].
| Antimicrobial | Our Study | Neelam et al.,., (Chandigarh) [1] | Dash M et al., ., (Odisha) [17] | Goel N et al.,., (Haryana) [18] | Tripathi et al.,., (Bhopal) [19] | Rit K et al.,., (kolkata) [20] |
|---|
| Resistance % | Resistance% | Resistance% | Resistance% | Resistance% | Resistance% |
|---|
| Cip | 75.64 | 26.8 | 86 | 89.7 | ND | ND |
| G | 82.72 | 20.1 | 70 | ND | ND | 70.3 |
| Cefo | 80.54 | 22.3 | 93 | 100 | 100 | 74.1 |
| Ak | 88.95 | 25.4 | 61 | 87.2 | 55 | 14.29 |
| Imp | 15.21 | 67.4 | 19 | 25.6 | 43 | 5.2 |
| PT | 32.97 | 61.6 | 23 | ND | 86.92 | 18,2 |
Cip:Ciprofloxacin G:Gentamicin Cefo:Cefotaxime
Ak:Amikacin Imp:Imipenem PT:Piperacillin –Tazobactam ND: Not done
Resistance pattern observed by us in our study was similar to the studies described in the above table [1,17,18]. The differences observed between the studies can be due to the methods and resistance patterns which are influenced by environmental factors and antimicrobial susceptibility patterns used [20]. Thus by observing the above table it can be said that majority of the isolates from almost all studies were resistant to commonly used antibiotics such as Ceftazidime, Gentamicin, Amikacin, Ciprofloxacin, Ampicillin and the list continues. This means that MDR isolates are increasing due to unchecked use of antibiotics.
Conclusion
Today Acinetobacter species can be considered a global threatening nosocomial infection and had emerged as a trouble maker for the clinicians. We have limited our research study to a recent period since this bacteria might be evolving overtime. Its remarkable growth in resistance to first and second line of antibiotics emphasises in giving it a lot of importance. There is an emerging need for identification of the genes and mechanisms for multidrug resistance else we may soon be facing end of antibiotic era. Otherwise the list of appropriate antibiotics will be shortened owing to its remarkable property of resistance.
Cip:Ciprofloxacin G:Gentamicin Cefo:CefotaximeAk:Amikacin Imp:Imipenem PT:Piperacillin –Tazobactam ND: Not done
[1]. Taneja N, Singh G, Singh M, Sharma M, Emergence of tigecycline and colistin resistant Acinetobacter baumanii in patients with complicated urinary tract infections in north IndiaIndian J Med Res 2011 133(6):681-4. [Google Scholar]
[2]. Falagas ME, Bliziotis IA, Siempos II, Attributable mortality of Acinetobacter baumannii infections in critically ill patients:a systematic review of matched case –cohot studiesCrit Care 2006 10:R48 [Google Scholar]
[3]. Anitha P, Ramaiah S, Beta lactamase in Acinetobacter Species: A Global ThreatIJPBS 2011 2(4) [Google Scholar]
[4]. Aslan M, Klompas M, Acinetobacter baumannii:An emerging and important pathogenJCOM 2010 17(8):363-7. [Google Scholar]
[5]. Wright Gerard D, Sutherland D, New Strategies for combating multidrug-resistant bacteriaCanada Trends in Molecular Medicine 2007 13(6):260-7. [Google Scholar]
[6]. Falagas Matthew E, Koletsi Patra K, Bliziotis Loannis A, The diversity of definitions of multidrug-resistant and pandrug-resistant Acinetobacter baumannii and pseudomonas aeruginosaJ Med microbiol 2006 55(12):1619-29. [Google Scholar]
[7]. Appleman MD, Belzberg H, Citron DM, Heseltine PN, Murray Yellin A, Berne TV, In vitro activities of non-traditional antimicrobials against multidrug resistant Acinetobacter baumannii strains isolated in an intensive care unit outbreakAntimicrob Agents Chemothe 2000 44:1035-40. [Google Scholar]
[8]. Kuo LC, Teng LJ, Yu CJ, Ho SW, Hsueh PR, Dissemination of a clone of unusual phenotype of pandrug-resistant Acinetobacter baumannii at a university hospital in TaiwanJ Clin Microbiol 2004 42:1759-63. [Google Scholar]
[9]. Tiwari V, Kapil A, Mohanty RR, Carbapem-hydrolysing oxacillinase in high resistant strains of Acinetobacter baumannii isolated from IndiaMicrobial pathogenesis 2012 55(2):81-6. [Google Scholar]
[10]. Neonakis Ik, Spandidos DA, Petinaki E, Confronting multidrug- resistant Acinetobacter baumannii:a reviewInt J Antimicrob Agents 2011 37(2):102-9. [Google Scholar]
[11]. Bush Karen, Jacoby George A, Minireview Updated Functional Classification of Beta lactamasesAmerican society for microbiology 2010 54(3):969-76. [Google Scholar]
[12]. Ambler RP, Structure of BetalactamasesPhillos Trans R Soc Lond B Biol Sci 1980 289(1036):321-31. [Google Scholar]
[13]. Livermore David M, Beta lactamases in laboratory and clinical resistanceClinical microbiology reviews 1995 8(4):557-84. [Google Scholar]
[14]. Alireza Japoni-Nejad, Sofian M, Belkum A, Ghaznavi-Rad Nosocomial outbreak of extensively and Pan Drug- Resistant Acinetobacter baumannii in Tertiary hospital in Central part of IranJundishapur Journal of microbiology 2013 6(8):e9892 [Google Scholar]
[15]. Irfan S, Metallo beta lactamase producing clinical isolates of Acinetobacter species and Pseudomonas aeruginosa from intensive care unit of tertiary care hospitalIndian J Microbiol 2006 26(3):243-5. [Google Scholar]
[16]. Luzzaro F, Mezzatesta M, Mugnaioli C, Perilli M, Stefani S, Amicosante G, Trends in production of extended-spectrum beta-lactamases among enterobacteria of medical interest: report of the second Italian nationwide surveyJ Clin microbial 2006 44(5):1659-64. [Google Scholar]
[17]. Dash M, Padhi S, Pattnaik S, Mohanty I, Misra P, Frequency, risk factors, and antibiogram of Acinetobacter species isolated from various clinical samples in a tertiary care hospital in Odisha, IndiaAvicenna J Med 2013 3(4):97-102. [Google Scholar]
[18]. Goel N, Chaudhary U, Aggarwal R, Bala K, Antibiotic sensitivity pattern of gram negative bacilli isolated from the lower respiratory tract of ventilated patients in the intensive care unitIndian J Crit Care Med 2009 13(3):148-51. [Google Scholar]
[19]. Tripathi C P, Gajbhiye R S, Agarwal N G, Adv Clinical and antimicrobial profile of Acinetobacter spp. An emerging nosocomial superbugBiomed Res 2014 3:13 [Google Scholar]
[20]. Rit K, Saha R, Multidrug-resistant acinetobacter infection and their susceptibility patterns in a tertiary care hospitalNiger Med J 2012 53:126-8. [Google Scholar]