Congenital Heart Disease (CHD), either due to septal defect or great vessel anomaly is more prone for hypoxia [1,2]. Chronic hypoxia of CHD results in a down-regulation of antioxidant defenses, making cells vulnerable to oxidative damage [3]. The oxidative damage and impaired antioxidant defense results in an imbalance between the amount of oxygen supplied to the cell and the amount required which subsequently leads to the single strand breaks and oxidative DNA damage [4,5]. HIF-1 and p53 induced by hypoxia activates the pro-apoptotic factors which lead to the alteration in regulation of pro-apoptotic gene Blc-2 to be also involved in causing the DNA damage [3]. The hemodynamic disturbances in CHD depend on the nature of the anatomical defects involved [1]. Studies regarding DNA damage through comet assay on individuals with Tetralogy of Fallot (TOF) [3] and on children with all types of cardiac anomalies [2] showed a positive indication of oxidative DNA damage. The biochemical parameters of oxidative stress and DNA damage co-exist; the former has been studied intensively while in the latter no attempt was made with reference to the type/s of cardiac anomaly. Hence the present study was conducted to find out the DNA damage in children with isolated septal defect and septal defect with great vessel anomaly of heart and to compare the same.
Materials and Methods
Study Subjects
This case-control study on children with congenital heart disease was conducted in the Cytogenetic laboratory, Department of Anatomy, JIPMER in collaboration with the Departments of Paediatrics after obtaining the approval from JIPMER Scientific Advisory Committee and Institute Human Ethical Committees. Forty children with congenital heart disease were diagnosed clinically and confirmed by echocardiography, were included as cases and age and gender matched healthy children were included as the control group in the current study. The cases were categorized broadly into children with isolated septal defects and those with septal defects associated with great vessel anomaly based on the echocardiogram [Table/Fig-1a,1b] and [Table/Fig-2a,2b]. Written Informed Consent was obtained from the concerned parents pertaining to the investigation.
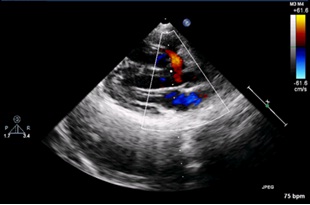
2D color Doppler showing VSD (Isolated Septal Defect) with left to right shunt
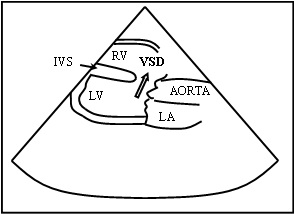
Schematic representation of 2D color Doppler showing VSD with left to right shunt LA – Left Atrium, RV – Right Ventricle, LV – Left Ventricle, VSD – Ventricular Septal Defect, IVS – Inter Ventricular Septum
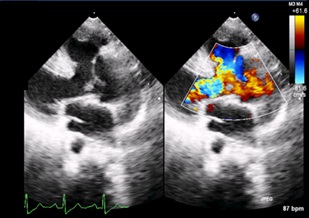
Parasternal long axis view 2D Color Doppler showing VSD and Aortic Override (Septal Defect with Great Vessel Anomaly)
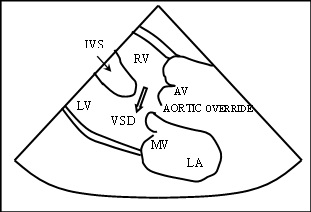
Schematic representation of Parasternal long axis view 2D Color Doppler showing VSD and Aortic Override LA – Left Atrium, RV – Right Ventricle, LV – Left Ventricle, VSD – Ventricular Septal Defect, MV – Mitral Valve, IVS – Inter Ventricular Septum,, AV – Aortic Valve
Sample Collection
One ml of peripheral venous blood was collected through heparinized syringe under strict aseptic conditions and isolation of lymphocytes was done by Density Gradient Separation Technique for performing comet assay.
Comet Assay
Widely accepted and conventionally followed protocol for Comet Assay [6,7] was performed on the blood samples collected. Lymphocytes isolated from the peripheral whole blood by using histopaque were suspended in low melting agarose which was transferred gently on glass slide pre-coated with normal melting agarose by using an identical sized coverslip for achieving uniform dispersion of cells over the pre-coated slides. The slides were transferred to refrigerator for few minutes and covered again with a fresh layer of normal melting agarose layer after removing the coverslip. The slides were then transferred to chilled lysis solution for overnight and were then subjected to electrophoresis in an alkaline buffer of pH 10 by using 300mA for 30 minutes. After neutralization with TRIS buffer followed by fixation with glycerol, Tri Chloroacetic Acid (TCA) and Zinc Sulphate (ZnSo4) the slides were stained with the working solution of 60microgram of silver nitrate in 300ml of distilled water. The slides stained with silver nitrate were observed under a bright-field light microscope Olympus-BX 51-Japan for comets and the images were captured using CCD camera. The captured images of the comets were later analyzed using comet software–TritekcometscoreTM version 1.5.
Scoring of Comets
Fifty comets of various dimensions were selected randomly from each slide at 20 X magnification for analysis. The captured images [Table/Fig-3,4and5] were analyzed by using comet software. The Comet parameters that were measured and analyzed in the current study were-Total comet length (TCL), Head diameter (HD), % of DNA in head (%DH), Comet tail length (TL) and % of DNA in tail(%DT).
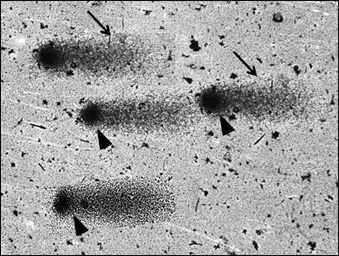
Comets – Isolated Septal Defect
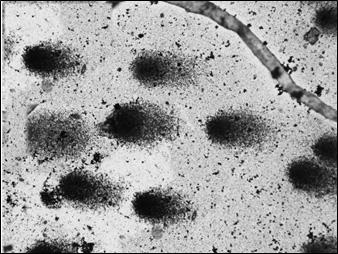
Comets – Septal Defect with Great Vessel Anomaly Black arrow heads – comet head, Black arrows – comet tail
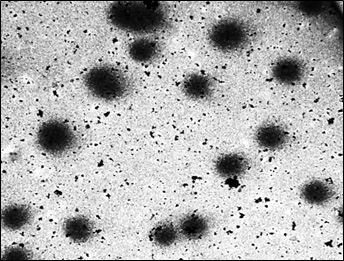
Statistical Analysis
The parametric Unpaired t-test through SPSS-version 19 statistical software towards achieving the threshold significance of p<0.05 was followed.
Results
The distribution of cases among the study group is shown in the [Table/Fig-6], of the total cases of ISD, VSD alone comprised of 65%; while 70% of TOF in SD-GVA group [Table/Fig-6]. ISD with equal number of appropriately selected controls showed statistically significant data of comet metrics with regards to TCL, HD, TL, %DH and %DT with the p-value <0.05 [Table/Fig-7].Similar significant difference was also observed in the other with reference to all parameters of comet except %DH [Table/Fig-8].While correlating the ISD with SD–GVA group, the degree of damage was found to be much greater in SD-GVA group with reference to all comet metrics [Table/Fig-9].
Distribution of CHD among cases ISD – Isolated Septal Defect, SD with GVA – Septal Defect with Great Vessel Anomaly, ASD – Atrial Septal Defect, VSD – Ventricular Septal Defect, PDA – Patent Ductus Arteriosus, TOF – Tetralogy of Fallot
| Type of CHD | Number | Percentage |
|---|
| Isolated Septal Defect (ISD) (Acyanotic) (n=20) | VSD | 13 | 65 |
| ASD | 04 | 20 |
| VSD with ASD | 03 | 15 |
| Septal Defect with Great Vessel Anomaly (SD with GVA) (n=20) | TOF (Cyanotic) | 14 | 70 |
| VSD with PDA | 03 | 15 |
| ASD with PDA | 03 | 15 |
Comparison of Comet metrics between children with isolated septal defect of heart and Controls. All Comet metrics were expressed in mean ± SD
| Study Group | Total Comet Length (μm) | Head Diameter (μm) | Comet Tail Length (μm) | % DNA in Head | % DNA in Tail |
|---|
| ISD (n=20) | 69.20±15.15 | 49.53±10.66 | 19.69±12.13 | 88.29±3.68 | 11.71±3.68 |
| Control n=20) | 30.08±5.31 | 29.32±5.27 | 0.79±1.70 | 88.67±7.70 | 10.50±8.11 |
| p-value | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
Comparison of Comet metrics between children with septal defect associated with great vessel of heart and Controls. All Comet metrics were expressed in mean ± SD
| Study Group | Total Comet Length (μm) | Head Diameter (μm) | Comet Tail Length (μm) | % DNA in Head | % DNA in Tail |
|---|
| SD with GVA (n=20) | 78.16±20.53 | 53.31±10.86 | 29.02±17.96 | 85.58±5.67 | 18.57±5.00 |
| Control (n=20) | 30.42±5.44 | 29.46±5.27 | 1.04±1.70 | 88.67±7.70 | 11.33±7.70 |
| p-value | p < 0.05 | p < 0.05 | p < 0.05 | p > 0.05 | p < 0.05 |
Comparison of Comet metrics between children with isolated septal defect and septal defect associated with great vessel of heart. All Comet metrics were expressed in mean ± SD
| Study Group | Total Comet Length (μm) | Head Diameter (μm) | Comet Tail Length (μm) | % DNA in Head | % DNA in Tail |
|---|
| ISD (n=20) | 69.20±15.15 | 49.53±10.66 | 19.69±12.13 | 88.29±3.68 | 11.71±3.68 |
| SD with GVA (n=20) | 78.16±20.53 | 53.31±10.86 | 29.02±17.96 | 85.58±5.67 | 18.57±5.00 |
| p-value | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
Discussion
The oxidative stress markers that commonly measured are malonyldialdehyde, plasma catalase, total antioxidant status, total oxidant status and oxidative stress index.There is a difference of opinion with reference to the level of oxidative stress between cyanotic and acyanotic CHD. Ercan et al., [5] are of the opinion that the oxidative stress estimated through biochemical means was marginally increased when compared to controls in VSD - acyanotic [5]. The same phenomenon was observed in the current series of ISD which includes VSD, ASD and VSD with ASD with reference to data of TCL, HD, TL, %DH and %DT. Similar observation of statistically significant levels of DNA damage was also seen in the other group which comprises of SD with GVA– which includes TOF – with cyanosis, VSD with PDA and ASD with PDA.
The DNA damage seen/ calibrated appears to be more extensive and severe in SD with GVA when compared with cases exclusively of ISD. The data is agreeing with the findings of oxidative stress estimated by Srujana et al., [3] with reference to the comet tail length in TOF and biochemically estimated oxidative stress put forth by Ercan et al., [5] for VSD and TOF. Therefore, it is apparent as the severity of the disease progress in terms of complexity of the structural defects of cardiac playing a major role for altered configuration of comets in cases investigated expressed in terms of increase in DNA damage.
The results of the present study are also in agreement with the findings of Rokicki et al., [8] with regards to the oxidant and anti-oxidants levels in infants and neonates less than one year. The anti-oxidants measured by earlier workers were glutathione peroxidase, catalase, superoxide dismutase, Vitamin E, uric acid and selenium and the oxidant- MDA. Of which glutathione peroxidase and MDA showed an imbalance between them in cases of cyanotic heart disease. In addition, both cyanotic and acyanotic cases showed very low levels of circulating Vitamin-E (anti-oxidant) [8]. Though the methodology for estimation of the oxidative stress is different in the current study, the level of oxidative stress was found to be consistent with that of Rokicki et al., [8]; with regards to DNA damage caused.
Conclusion
To conclude, there is a strong evidence of hypoxia induced DNA damage in individuals of isolated septal defect and septal defect associated with great vessel anomaly of the heart in cases investigated with profound damage in the later.