Evaluation of Myofibroblasts by Expression of Alpha Smooth Muscle Actin: A Marker in Fibrosis, Dysplasia and Carcinoma
Bharath Rao K1, N. Malathi2, Sangeetha Narashiman3, Sharada T Rajan4
1 Assistant Professor, Department of Oral Biology, Faculty of Dentistry, Melaka Manipal Medical College (MMMC), Manipal University, Karnataka, Manipal, India.
2 Professor and Head, Department of Oral Pathology and Microbiology, Sri Ramachandra Dental College, Sri Ramachandra University, Chennai, India.
3 Assistant Professor, Department of Oral Pathology and Microbiology, Sri Ramachandra Dental College, Sri Ramachandra University, Chennai, India.
4 Assistant Professor, Department of Oral Pathology and Microbiology, Sri Ramachandra Dental College, Sri Ramachandra University, Chennai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr Bharath Rao K, Assistant Professor, Department of Oral Biology, Faculty of Dentistry, Melaka Manipal Medical College (MMMC), Manipal University, Udupi District, Karnataka, Manipal - 576104, India.
Phone: +919972414678,
E-mail: bharathraok@yahoo.com, Fax no: +08202571905
Objective: Evaluation of Myofibroblasts by studying expression of Alpha smooth muscle actin: A marker of Fibrosis, Dysplasia and Carcinoma.
Background: Myofibroblasts are cells that have contractile properties and are involved in inflammation, wound healing, fibrosis and oncogenesis in most of the organs and tissues. They are involved in healing and granulation tissue formation which occur after tissue injuries, also produce inflammatory mediators, growth factors and help in extracellular matrix reorganization by secretion of proteins like collagen, fibronectin, etc. Because of their component, Alpha smooth muscle actin ([alpha]-SMA), they are involved in the contraction of extracellular matrix and aid in tissue contraction. The myofibroblasts disappear by apoptosis after completion of repair, but their persistence causes a dysfunction in the repair mechanism, leading to excessive contraction and extracellular matrix (ECM) secretion and thus, fibrosis. The purpose of this study was to evaluate the presence of myofibroblasts in cases of Oral Submucous fibrosis (OSMF), which consisted of very early, early and moderately advanced OSMF, OSMF with dysplasia and oral squamous cell carcinoma (OSCC), by detecting (alpha)-SMA, which is a specific marker for myofibroblasts.
Materials and Methods: The study sample consisted of three groups which comprised of 41 cases of OSMF, 10 cases of OSMF with dysplasia and 11 cases of OSCC. All the cases were subjected to immunohistochemistry by using (alpha)-SMA antibody for detection of myofibroblasts.
Results: The presence of myofibroblasts was significantly higher in oral squamous cell carcinomas as compared to that in OSMF with dysplasia and OSMF. A statistical significance was also noted between the staining index and age of the individuals and the staining index and duration of the habit.
Conclusion: Myofibroblasts play a role in fibrosis, as was seen in OSMF. Activated myofibroblasts secrete proteolytic enzymes and cause matrix degradation, which is instrumental in cancer cell invasion and metastasis. Further studies in which the myofibroblasts are targetted, may help in providing therapeutic regimens in fibrosis, dysplasia and cancer.
Oral submucous fibrosis, Oral squamous cell carcinoma, Alpha smooth muscle actin
Introduction
Myofibroblasts are contractile cells. They are involved in morphogenesis, inflammation, wound healing, fibrosis and oncogenesis in many tissues and organs. These cells are found in normal tissues and they increase in number during the process of wound healing. They also play a role in creating granulation tissue. Myofibroblasts help in ECM reorganization by the production of numerous inflammatory mediators, growth factors and proteins of the extracellular matrix, like collagen, fibronectin, etc. The mediators which are produced by the mast cells play a role in the regulation of myofibroblast differentiation and function. The myofibroblasts cause contraction of ECM due to their component of (alpha)-SMA and aid in tissue contraction.They disappear by apoptosis after completion of the repair process, but their sustained presence stimulates dysfunctional repair mechanisms, causing excess contraction, extracellular matrix secretion, and thus, fibrosis. This has been implicated in scleroderma, hypertrophic scars, heart, lung and liver fibrosis and Dupytren’s disease [1]. TGF-β, which is a potent proinflammatory and pro-fibrotic cytokine, is the main trigger for increased collagen production and decreased degradation pathways, as is seen in OSMF [2]. Increased expression of α ν β6 integrin in OSMF promotes myofibroblast differentiation by activation of TGF-β1 [3]. TGF-β is thus intimately involved in the differentiation of fibroblasts to myofibroblasts, which is characterized by the presence of (alpha)-SMA containing stress fibres. Cell culture experiments show a direct cell-cell contact between fibroblasts and keratinocytes, which is important for TGF-β activation and initiation [4]. Increased expression of myofibroblasts in OSMF may suggest that myofibroblast production and persistence could be one of the mechanisms by which TGF-β induces a fibrotic response in OSMF [5]. Cytoskeleton proteins, ECM molecules, Cytokines and Nucleus proteins which are elements of Epithelial Mesenchymal Transition (EMT), which are commonly associated with OSMF, lead to the possibility of EMT being a part of fibrosis of oral mucous, which is triggered by betel quid chewing habit in OSMF [6]. Extensive myofibroblast differentiation requires close contact between keratinocytes and fibroblasts, which suggests their role in cellular signaling in dermal wounds [7].
Studies have shown that the stroma of neoplastic tissues plays an important role in tumour progression. With the conversion of non diseased epithelial tissue to pre-cancerous epithelium to carcinoma, changes occur, from normal to ‘primed’ to ‘activated or tumour’, which are associated to the stroma [8,9]. Fibroblasts are one of the most important mesenchymal cell types in tumour progression [8, 10]. Transdifferentiation of fibroblasts into myofibroblasts is an early and crucial event in tumourigenesis and it is mediated by cytokines and growth factors which are expressed by tumour cells [8–11].
The induction of myofibroblasts by OSCC derived factors, that in turn promote carcinomatous proliferation, leading to neoplastic growth, has been demonstrated [12,13]. Studies have been conducted individually to evaluate the role of myofibroblasts in OSMF and OSCC. This was the first study which compared the distribution of myofibroblasts in OSMF, OSMF with dysplasia and OSCC by using (alpha)-SMA, which has been suggested to be the most reliable marker of myofibroblast differentiation.
Materials and Methods
The study samples included 62 formalin fixed, paraffin embedded tissue blocks which were retrieved from the archives of Sri Ramachandra Dental College, Chennai, based on the medical records of the patients, which included age, sex and durations of habits. Diagnosis was confirmed by two oral pathologists by using haematoxylin and eosin stained sections. The study group consisted of 41 prediagnosed cases of OSMF (25 cases of very early and early OSMF and 16 cases of moderately advanced OSMF, according to Pindborg and Sirasat [14]) , 10 cases of OSMF with dysplasia and 11 cases of OSCC. The study was performed over a duration of 8 months. The sections were subjected to immunostaining with (alpha)-SMA.
The primary antibody immunohistochemical kit was procured from Abcam Plc, Cambridge, UK, in its concentrated form. It was placed in an Eppendorf tube, centrifuged at 12,000 rpm for 20 seconds and later used with Tris buffer in a dilution of 1/100. The immunostaining for (alpha)-SMA was performed in a two step process that involved the demonstration of antigens in tissues and cells first, by the binding of an antibody to the antigen of interest, followed by detection and visualization of the bound antibody by an enzyme chromogenic system. To evaluate the specificity of the immunoreactions, known positive and negative tissue controls were used.
Immunohistochemical Analysis
Stromal spindle cells which were positive for (alpha)-SMA were regarded as myofibroblasts. The staining was assessed by the evaluation of staining intensity and the percentage of (alpha)-SMA Positive cells according to the criteria which were modified from Etemad-Moghadam et al., [15] and Angadi et al., [5]. The percentage of immuno-positive cells seen among the non-inflammatory and non-endothelial stromal cells present in the sub epithelial connective tissues of OSMF and OSMF with dysplasia, was recorded. In OSCC, the immune-positive cells which were adjacent to the carcinomatous islands were recorded. The percentage was recorded as 0% = no positive cells, 1%=1-25% positive cells, 2% - 26-50% positive cells, 3% = 51-75% positive cells and 4% = 76-100% positive cells. The staining index was recorded as zero (0), low (1), moderate (2), high (3) and intense (4). All the sections were evaluated by two observers to eliminate inter-observer bias and any disagreements which occurred were resolved by using a five headed microscope.
Statistical Analysis
The differences in the presence of myofibroblasts between the groups were analyzed by using the Pearson’s Chi-square test with Yates’ continuity or Fischer’s exact test (2-tailed). P-values of < 0.05 was considered to be statistically significant.
Results
A positive reaction for (alpha)-SMA was observed. The leiomyosarcoma tissue that served as a positive control exhibited positivity for (alpha)-SMA. This proved that the antigenic expression was properly maintained in the study sample.
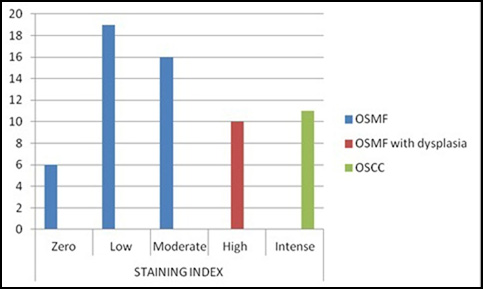
The staining index was found to be intense in all the cases of OSCC [Table/Fig-1,2] , high in the cases of OSCC with dysplasia, moderate in 16 cases of OSMF and low in 19 cases of OSMF [Table/Fig-3]. Six cases of OSMF demonstrated no staining.
Islands of squamous cell carcinoma showing (alpha)-SMA expression signifying myofibroblasts
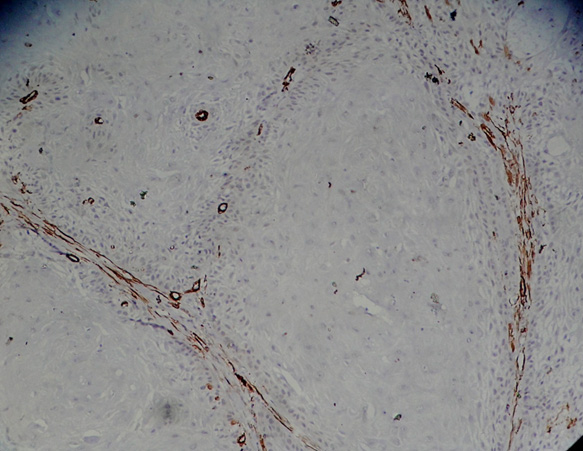
Islands of squamous cell carcinoma showing (alpha)-SMA expression signifying myofibroblasts; high power view (X40)
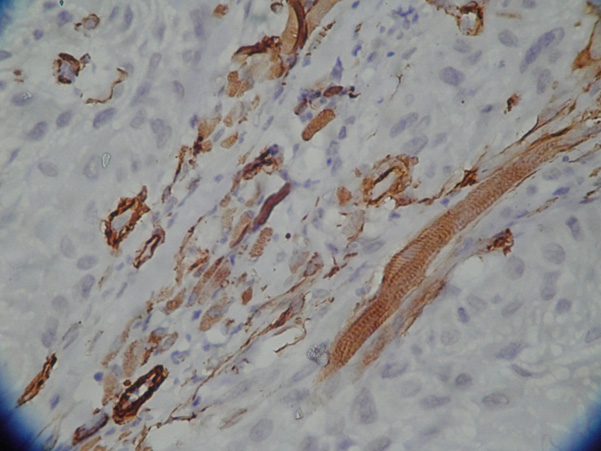
(alpha)-SMA expression signifying myofibroblasts in oral submucous fibrosis low power view (X10)
There was no statistical significance between the sex of the individual and staining index. However, a statistical significance was noted between the staining index
and age of the individual [Table/Fig-4].
and duration of the habit [Table/Fig-5].
among the three study groups [Table/Fig-6] [Table/Fig-7].
Association between age and staining index
| Variable | Category | Staining index | P-Value |
|---|
| Zero | Low | Moderate | High | Intense |
|---|
| No. of cases | % | No.of cases | % | No.of cases | % | No.of cases | % | No.of cases | % |
|---|
| Age | <30 | 2 | 33.3 | 9 | 47.4 | 10 | 62.5 | 2 | 20.0 | 1 | 9.1 | 0.001 |
| 30-49 | 3 | 50.0 | 7 | 36.8 | 4 | 25.0 | 6 | 60.0 | 1 | 9.1 |
| 50+ | 1 | 16.7 | 3 | 15.8 | 2 | 12.5 | 2 | 20.0 | 9 | 81.8 |
Association between duration of habits and staining index
| Variable | Category | Staining index | P-Value |
|---|
| Zero | Low | Moderate | High | Intense |
|---|
| No. of cases | % | No.of cases | % | No.of cases | % | No.of cases | % | No.of cases | % |
|---|
| Age | Nil | 1 | 16.7 | 4 | 21.1 | 3 | 18.8 | 5 | 50.0 | 8 | 72.7 | 0.01 |
| < 5 years | 2 | 33.3 | 10 | 52.6 | 8 | 50.0 | 1 | 10.0 | 0 | 0.0 |
| 5-10 years | 2 | 33.3 | 3 | 15.8 | 4 | 25.0 | 0 | 0.0 | 1 | 9.1 |
| >10 years | 1 | 16.7 | 2 | 10.5 | 1 | 6.3 | 4 | 40.0 | 2 | 18.2 |
Comparison of staining index among different groups
| Staining Index | Oral submucous fibrosis | Oral submucous fibrosis with dysplasia | Oral squamous cell carcinoma | P – value |
|---|
| No. of cases | % | No.of cases | % | No.of cases | % | <0.0001 |
|---|
| Zero | 6 | 14.6 | 0 | 0.0 | 0 | 0.0 |
| Low | 19 | 46.3 | 0 | 0.0 | 0 | 0.0 |
| Moderate | 16 | 39.0 | 0 | 0.0 | 0 | 0.0 |
| High | 0 | 0.0 | 10 | 100.0 | 0 | 0.0 |
| Intense | 0 | 0.0 | 0 | 0.0 | 11 | 100.0 |
Comparison of staining index among different groups
Discussion
Myofibroblasts are found in humans in normal tissues such as lymph nodes, blood vessels, uterine submucosa, intestinal villous core, lung septa and testicular stroma [16] and in reactive lesions, locally aggressive fibromatosis, benign tumours and sarcomas, which show myofibroblast differentiation [17].
Only few studies have compared the ages of the patients with the staining indices of (alpha)-SMA of myofibroblasts. In this study, a statistically significant difference was seen with respect to the ages of the patients and the staining indices. The increase in number of myofibroblasts with age could be caused by fibrosis, which is a complication of tissue regeneration failure and ineffective epithelial repair, in organs which have an age dependant increase in mammalian tissue [18]. Louise et al., stated that fibrosis could be an adaptive response which was shown by mesenchymal cells, by secreting a provisional matrix to help in re-epithelialization, to restore tissue barrier function; but, a persistent, failed re-epithelialization and a persistent mesenchymal activation can result in a sustained and progressive fibrosis [19].
The habits included chewing of quid with betel leaves, areca nut, tobacco and lime, mawa and Pan Masala. The durations of the habits, which were recorded as less than 5 years, 5 to 10 years and longer than 10 years, were statistically significant with respect to the staining indices. The quid was placed for about 15 minutes to an hour in the buccal vestibule and this process was repeated for six to seven times times a day, which caused constant irritation to the oral tissues. The coarse fibres of areca nut cause mechanical irritation to the mucosa, and they facilitate diffusion of the contents into the sub-epithelial connective tissue [20]. Cytokines including TGF-β may be involved in the transdifferentiation of fibroblasts to myofibroblasts [21]. It has been accepted that TGF-β has a key role in inducing the differentiation of myofibroblasts in lesions with high levels of inflammatory mediators, which could be the case in OSMF. Areca nut habit with or without use of tobacco is important in the development of oral squamous carcinoma . The use of paan without the use of tobacco is also associated with squamous cell carcinoma [22]. The number of myofibroblasts were increased in OSCC, as was concluded by Etemad-Moghadam et al., [15].
A statistically significant difference was observed in the three groups in relation to the staining indices. The number of (alpha)-SMA positive myofibroblasts showed a progressive increase from OSMF to OSMF with dysplasia to OSCC. Over the past two decades, numerous studies have been conducted on the roles of the constituents of areca nut in the pathogenesis of OSMF. OSMF can be considered as a collagen metabolic disorder [23]. TGF-β plays a major role in wound repair, fibrosis and in the development of many fibrous diseases [24]. It increases the synthesis of collagen and decreases its degradation by stimulating various inhibitory mechanisms and thus, causing deposition of extracellular matrix. It is also essential for healing, but its overproduction leads to scar tissue and fibrosis [25]. TGF-β induces the differentiation of fibroblasts to myofibroblasts and it promotes survival of myofibroblasts by providing protection against apoptosis by inhibiting iNOS ( Nitrous oxide synthetase) induction and reducing BCL-2 expression [26]. The incidence of myofibroblasts in the cases of OSMF in this study, suggested that OSMF could be considered as a failed wound healing process of the oral mucosa, caused by chronic injury, which could lead to scarring and fibrosis [27]. Some of the cases of OSMF demonstrated an increase in the number of myofibroblasts, which could be related to the histologic stage of OSMF, as was concluded by Angadi et al., [5].
In our study, all the cases of OSCC demonstrated intense staining and OSMF with dysplasia showed high staining indices, which indicated that (alpha)-SMA expression in the stroma of OSCC was greater than that in OSMF with dysplasia and that it was in accordance with the role of (alpha)-SMA positive myofibroblasts in invasive behaviour of OSCC, as was stated by Safora Seifi et al., They studied (alpha)-SMA expression in squamous epithelial carcinoma, oral dysplasia and hyperkeratosis and demonstrated an increased presence of (alpha)-SMA in carcinoma as compared to that seen in oral dysplasia and hyperkeratosis [28]. Mareilena Vered et al. studied the expression of (alpha)-SMA in cells at the tumour connective tissue interface in hyperplasia, mild dysplasia, moderate-to-severe dysplasia and in human tongue carcinoma. TGF-β assessment and double staining with epithelial membrane antigen and (alpha)-SMA was done only in cases of carcinoma. The myofibroblasts were increased in number in the cases of carcinoma and they were more in number than those seen in dysplasia and hyperplasia. TGF-β was expressed in 95% cases of carcinoma and epithelial membrane antigen and (alpha)-SMA positive cells were seen in 41% of the cases . These cells may be a manifestation of epithelial-mesenchymal transition and they could serve as a source of myofibroblasts. These findings could be linked to the expression of transforming growth factor beta in malignant cells [29] and they could be correlated with our findings in cases of OSMF with dysplasia, as TGF-β is known to play a major role in the molecular pathogenesis of the disease [9]. Etemad Moghadam et al., used (alpha)-SMA, vimentin and desmin markers on 40 samples of OSCC, 15 cases of dysplasia and 15 cases of normal oral epithlieum and concluded that the presence of myofibroblasts in the stroma of oral cancer was an expression of their key roles in carcinogenesis [15]. The expressions of CD34, (alpha)-SMA and TGF-β1 were studied in squamous intraepithelial lesions and in SCCs of the larynx and the hypopharynx. It was concluded that the appearance of (alpha)-SMA positive myofibroblasts and disappearance of CD-34 positive stromal cells wereassociated with the transformation of laryngeal squamous intraepithelial lesions to SCCs. The pattern of stromal reaction was found in the tumour and the peritumoral zone. These findings support the fact that overproduction of TGF-β1 in carcinoma cells mediate one of the mechanisms of transformation of stromal cells to myofibroblasts in laryngeal carcinogenesis [30].
Summary and Conclusion
The limitations of this study include the fact that there was no way to determine whether the examined cases of OSMF with dysplasia would have remained as dysplastic entities or whether they would have transformed into OSCC and we were also unable to obtain enough samples of OSMF with dysplasia.
Myofibroblasts are a prominent finding in the tumour stroma of many but not all OSCC cases. Additional investigations such as tissue culture studies would be required to ascertain this fact and they may help in clarifying the roles of myofibroblasts in fibrosis, dysplasia and carcinoma and their contribution to carcinogenesis. If our results are confirmed by doing further investigations, then it could be possible to target the myofibroblasts and to develop a therapeutic regimen to prevent uncontrolled fibrosis seen in OSMF, as it is well known to have a cancerous potential.
[1]. Hinz B, Phan SH, Thannickal VJ, The myofibroblast: one function, multiple originsAm J Pathol 2007 170:1087-816. [Google Scholar]
[2]. Rajalalitha P, Vali S, Molecular pathogenesis of oral submucous fibrosis - a collagen metabolic disorderJ Oral Pathol Med 2005 34:321-8. [Google Scholar]
[3]. Moutasim KA, Mirza D, Marsh D, Integrin βvβ6 promotes TGF-b1 dependent myofibroblastic transdifferentiation in oral submucous fibrosisHead and Neck Oncol 2009 1(Suppl 1):14 [Google Scholar]
[4]. Krieg Thomas, Abraham David, Lafyatis Robert, Fibrosis in connective tissue disease: the role of the myofibroblast and fibroblast-epithelial cell interactionsArthritis Research and Therapy 2007 9(Suppl 2):S4 [Google Scholar]
[5]. Punnya V, Angadi, Alka D. Kale, Seema Hallikerimath. Evaluation of myofibroblasts in oral submucous fibrosis: correlation with disease severityJ Oral Pathol Med 2011 40:208-13. [Google Scholar]
[6]. Hu Yanjia, Jian Xinchun, The role of epithelial-mesenchymal transition in oral squamous cell carcinoma and oral submucous fibrosisClinica Chimica Acta 2007 383:51-56. [Google Scholar]
[7]. Shephard P, Martin F, Smola-Hess S, Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous TGF-b and IL-1Am J Pathol 2004 164:2055-66. [Google Scholar]
[8]. Li X, Ling TY, Gao YJ, Effect of arecoline on the differentiation of myofibroblasts of oral mucosa (Abstract)Zhongua Kuo Qiang Yi Xue Za Zhi 2007 42:423-7. [Google Scholar]
[9]. Beacham DA, Cukierman E, Stromagenesis: the changing face of fibroblastic microenvironments during tumor progressionSemin Cancer Biol 2005 15:329-41. [Google Scholar]
[10]. Amatangelo MD, Bassi DE, Klein-Szanto AJ, Cukierman E, Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblastsAm J Pathol 2005 167:475-88. [Google Scholar]
[11]. Liotta LA, Kohn EC, The microenvironment of the tumour-host interfaceNature 2001 411:375-9. [Google Scholar]
[12]. De Wever O, Mareel M, Role of tissue stroma in cancer cell invasionJ Pathol 2003 200:429-47. [Google Scholar]
[13]. Kellermann MG, Sobral LM, Da Silva SD, Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosisHistopathology 2007 51:849-53. [Google Scholar]
[14]. Pindborg JJ, Sirasat SM, Oral Submucous fibrosisOral Surg, Oral Med, Oral Pathol 1966 22:764-9. [Google Scholar]
[15]. Etemad-Moghadam S, Khalili M, Tirgary F, Alaeddin M, Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinomaJ Oral Pathol 2009 38:625-43. [Google Scholar]
[16]. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA, Myofibroblasts and mechano-regulation of connective tissue remodelingNat Rev Mol Cell Biol 2002 3:349-63. [Google Scholar]
[17]. Fletcher CDM, Myofibroblastic tumors: an updateVerh Dtsch Ges Path 1998 82:75-82. [Google Scholar]
[18]. Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G, Incidence and prevalence of idiopathic pulmonary fibrosisAm. J. Respir. Crit. Care Med 2006 174:810-16. [Google Scholar]
[19]. Louise Hecker, Vittal Ragini, Jones Tamara, Jagirdar Rajesh, Luckhardt Tracy R, Horowitz Jeffrey C, NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injuryNature Medicine 2009 15:1077-81. [Google Scholar]
[20]. Chiang CP, Hsieh RP, Chen TH, High incidence of autoantibodies in Taiwanese patients with oral submucous fibrosisJ Oral Pathol Med 2002 3:402-9. [Google Scholar]
[21]. Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Tumour-derived TGF-b1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cellsBritish Journal of Cancer 2004 90:822-32. [Google Scholar]
[22]. Tuan TL, Nichter LS, The molecular basis of keloid and hypertrophic scar formationMol Med Today 1998 4:19-24. [Google Scholar]
[23]. Tilakaratne WM, Klinikowski MF, Saku Takashi, Peters TJ, Warnakulasuriya Saman, Oral submucous fibrosis: Review on aetiology and pathogenesisOral Oncology 2006 42:561-8. [Google Scholar]
[24]. Ghahary A, Shen YJ, Scott PG, Gong Y, Tredget EE, Enhanced expression of mRNA for transforming growth factor-b, type I and type III procollagen in human postburn hypertrophic scar tissuesJ Lab Clin Med 1993 122:465-73. [Google Scholar]
[25]. Desmoulière A, Factors influencing myofibroblast differentiation during wound healing and fibrosisCell Biol Int 1995 19:471-6. [Google Scholar]
[26]. Dabelsteen S, Christensen S, Gron B, Bardow A, Dabelsteen E, HGF is released from buccal fibroblasts after smokeless tobacco stimulationOral Oncol 2005 4:509-14. [Google Scholar]
[27]. Safora Seifi, Shahryar Shafaei, Ensieh Shafigh, Seid Mehdi Sahabi, Hamidreza Ghasemi, Myofibroblast Stromal Presence and Distribution in Squamous Epithelial Carcinomas, Oral Dysplasia and HyperkeratosisAsian Pacific J CancerPrev :11:359-64. [Google Scholar]
[28]. Marilena Vered, Irit Allon, Amos Buchner, Dan Dayan, Stromal Myofibroblasts accompany Modifications in the Epithelial Phenotype of Tongue Dysplastic and Malignant Lesions CancerMicroenvironment 2009 2:49-57. [Google Scholar]
[29]. Ochicha O, Pringle JH, Mohammed AZ, Immunohistochemical study of epithelial-myofibroblast interaction in Barrett metaplasiaIndian J Pathol Microbiol 2010 53:262-6. [Google Scholar]
[30]. Nika Kojc, Nina Zidar, Boris Vodopivec, Nina Gale, Expression of CD34, a–smooth muscle actin, and transforming growth factor B1 in squamous intraepithelial lesions and squamous cell carcinoma of the larynx and HypopharynxHuman Pathology 2005 36:16-21. [Google Scholar]