Anaemia is the most common disease and even more so in a tropical country like India. Although, the prevalence of anaemia is estimated at 9 per cent in countries with high development, in countries with low development the prevalence is 43 per cent [1]. It affects the various organs including the heart. It is one of the most common causes of hyper dynamic state of heart at rest. It affects the heart by impairing the O2 supply of myocardium [2], thus supply – demand myocardial mismatch causing myocardial ischemia or infarction [3]. A number of mechanisms are available to compensate for the decrease in O2 transport associated with anaemia. They include an increase in Cardiac Output (CO) and decrease in circulation time [4]. These cardiac disturbances persist as long as the anaemia is severe [2] and quite strikingly these changes can be rapidly reversed by partial correction of anaemia in almost every instance [1]. In severe long standing anaemia, cardiac dilatation and hypertrophy are naturally expected due to hyperdynamic state [5].
To substantiate this, ECG studies for cardiac disturbances have been made less frequently. There is a great diversity of opinion available in literature, on reports of ECG changes in anaemia [6,7]. Early reports have described a decrease in QRS amplitude, T wave flattening and minor degrees of atrioventricular (AV) conduction disturbances [8], but these have not been observed in more recent studies. Later studies have reported frequent non-specific ST-T wave changes [9]. It is not certain, however that these changes are more common in anemic patients [1]. The abnormalities may be proportional to the severity of anaemia [10], or show no correlation to Hb level [11]. Hence in the present study we intended to study the electrocardiographic changes in anemic population and to correlate ECG changes seen with increasing severity of anaemia, with respect to every 1gm% decrease in hemoglobin (Hb) level from 8 gm% onwards.
Materials and Methods
This study was conducted on selected hundred (male and female) anemic individuals of age group 18- 30 years, during September 2013 to December 2013. Patients attending medicine Out-Patient Department (OPD) diagnosed as anaemia, irrespective of etiology, in whom, there was Hb < 8gm% were randomly included in the study. Patients with history of smoking, alcoholism, diabetes, hypertension, clinically evident disturbances of the cardiovascular system, or of any extra-cardiac affection that may produce ECG changes, were excluded from the study. To avoid any possible effect of age on ECG, we chose the population only between 18-30 years. Ethical clearance was obtained from the institution. Informed consent was also obtained from the patients selected for the study. Hb level was estimated by semi-automated analyser which provided idea about severity of anaemia. All the patients were grouped according to Hb level. Then in the selected hundred anemic individuals, resting ECG was taken using electrocardiograph machine, in all the twelve leads and analysed for the specific anemic changes.
Statistical Analysis
ECG findings and varying severity of Hb level of each group were correlated using Pearson’s co-relation co-efficient and association was calculated using Chi-square test (SPSS version 11).
Results
Hundred patients were grouped based on their Hb level as Group 1 to Group 7, each group with the range of 1gm% Hb, beginning from 0gm% Hb to 8gm% Hb. There was no significant correlation between clinical history with varying severity of anaemia, also between peripheral smear picture and Hb level. There was significant correlation between Hb level and ECG changes. The ECG findings are tabulated in [Table/Fig-1].
ECG changes in different hemoglobin groups
| ECG changes |
|---|
| Tachycardia | LVH | STD | Tw |
|---|
| Group | N | n | % | n | % | N | % | n | % |
| Group 1 | 4 | 3 | 75 | 1 | 25 | 3 | 75 | 2 | 50 |
| Group 2 | 4 | 4 | 100 | 1 | 25 | 3 | 75 | 1 | 25 |
| Group 3 | 7 | 7 | 100 | 2 | 29 | 5 | 71 | 2 | 29 |
| Group 4 | 13 | 10 | 76 | 1 | 0.07 | 7 | 54 | 1 | 0.07 |
| Group 5 | 14 | 7 | 50 | 1 | 0.07 | 2 | 0.14 | 1 | 0.07 |
| Group 6 | 24 | 13 | 54 | 1 | 0.04 | 4 | 0.17 | 2 | 0.08 |
| Group 7 | 34 | 1 | 0.03 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chi-square test | x2 = 46.40 | x2 = 2.16 |
| p-value | p=0.0001(HS) | p=0.99(NS) |
N = Number of patients, n= Number of patients in sub group, LVH = Left ventricular hypertrophy, STD = ST depression, Tw = T wave changes, HS = Highly significant, NS = Non significant
We found that, 72% of patients were distributed in Group 5,6 and 7 (Hb 5-8gm%) and the remaining 38% were distributed in group 1,2,3 and 4 (Hb level 0-5gm%). As the Hb level decreased there were more percentage of patients having tachycardia, and the Chi-square association showed high significance. As the Hb level decreased there was more percentage of patients having ECG changes, although the Chi-square association was not significant. In Group 7 (Hb level 7-8gm%) no ECG changes were seen. In Group 5 and 6 (Hb level 5-7gm%) showed less percentage of patients with ECG changes. In Group 1, 2, 3 and 4 (Hb level 1-5gm%) showed increasing percentage of patients with ECG changes – 50-75% having ST depression, 29-50% T wave changes and 25-30% LVH. The ECG pattern with only ST depression was seen as in [Table/Fig-2], and with both ST depression and T wave inversion was seen as in [Table/Fig-3].
ECG changes in a case showing tachycardia with ST depression in lead I, II, III, aVF, V3-6
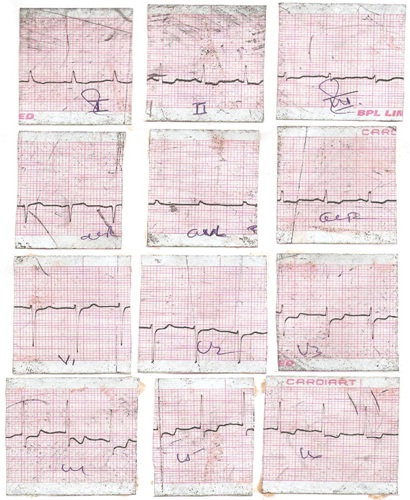
ECG changes in a case showing tachycardia with ST depression in Lead I, aVL, V4-V6 and T wave inversion in V4-V6
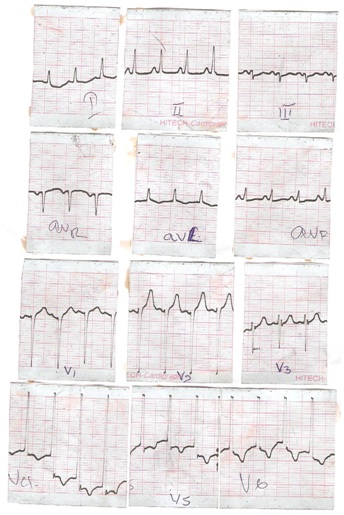
The correlation between the level of Hb and number of patients with tachycardia and ECG findings is shown in [Table/Fig-4].
Correlation between Haemoglobin level with ECG changes and tachycardia
| Parameters | Haemoglobin level |
|---|
| r | p |
| Tachycardia | -0.646 | 0.0001 |
| ECG changes | -0.572 | 0.0001 |
There is strong negative correlation between Hb level and tachycardia and ECG changes, indicating that as the Hb level decreases, there is an increase in occurrence of tachycardia and ECG changes.
Discussion
In the present study on 100 patients, the Hb level showed strong negative correlation with the ECG changes.
Tachycardia, seen in the present study seems to be a clinical evidence of physiological adjustments in circulation due to anaemia, as a compensatory increase in Cardiac output (CO), in order to maintain adequate O2 supply. Increase in CO can be achieved by increase in blood volume, preload, Heart Rate (HR) and stroke volume, along with a decrease in after load [12]. Literature shows that, though tachycardia contributes to higher CO than those with normal HR, no direct correlation between CO and HR could be established. However, it has been shown that, stroke volume is more closely related to elevated CO than tachycardia [13]. In a study, where in autonomic function were assessed in anemic patients, showed that they had low basal parasympathetic outflow to increase the HR as compensatory mechanism [14]. Hence we can say that tachycardia seen in anemic patients could be due to low basal parasympathetic outflow, to increase CO but doesn’t contribute much to the needed CO, unlike stroke volume, which was not included in our study, since we concentrated only on the ECG changes in anemic patients.
One of the ECG changes noted in our study was LVH, indicating cardiac enlargement. This was pointed out a century ago [15]. Cardiac enlargement without other etiologies has been observed more frequently in patients with anaemia, particularly in patients with low Hb [15]. In anaemia there is combination of increased HR and stroke volume, to increase CO, which in turn improves O2 delivery. To accommodate this greater output, there is an increase in LV chamber size, both systolic and diastolic [16,17]. The factor for cardiac enlargement could be both dilatation as well as hypertrophy. It seems that anaemia of shorter duration results in cardiac dilatation, whereas that of longer duration results in hypertrophy [15]. It was assumed that cardiac enlargement and hypertrophy in anemic patients was due to increased work of heart, but now it has been attributed to insufficient O2 supply to the myocardium [15]. It is interesting to note that cardiomegaly reportedly returns to normal within few weeks of resolution of anaemia [18].
The other changes seen in our study were ST segment depression and T wave flattening and inversion. Earlier studies have reported decreased QRS amplitude, T-wave flattening and minor degrees of atrioventricular conduction disturbances [8]. A study has also noted accentuation of T wave, appearances of Q wave in lead III, diminution and enlargement of QRS complex [11]. But, later many studies have reported changes in ECG like ST segment depression, flat or inverted T waves, but without corresponding changes in QRS complex [9,15,19,20].
In the present study such alterations in electrical conduction was seen in more per centage of patients with <5gm%, in patients with 5-7gm% very less percent of them showed such changes and in patients with Hb 7gm% there were no changes. Similar observations were made in a study of anemic patients with Hb level of 4-5gm% or less [15]. Yet another study has reported such findings in young patients, where Hb values were between 2.7gm% and 3.5gm% [15].
This could be because the ECG changes are not seen when the heart is in compensated state. But at 7gm%, there is a transition from a high-output (compensated) cardiac state to a state of LV dysfunction (decompensated). As the Hb level drops further, so does the LV function [21]. To substantiate this, few studies have shown elevated brain natriuretic peptide (BNP) levels in patients with clinical evidence of LV decomposition [22,23].
Though the changes in ECG are seen in more percentage of patients as the Hb level decreases, there was no association found in the present study. Similarly, no close parallelism was found between the degree of anaemia and that of cardiac disturbances in a study [11]. Experiments in animals have proved that, anaemia may produce ischemic disturbances of the myocardium. But ECG changes seen are not due to necrosis of heart muscle, but purely due to metabolic disturbances in myocardium resulting from O2 deficiency, caused by diminution of O2 – carrying power of the blood [11]. This could be the reason for not finding typical ischemic pattern in ECG of anemic patients. Unlike in ischemic pattern, the ECG changes due to anaemia, especially T- wave changes reverted back to normal within a week of correction of anaemia [19].
The difficulty of diagnosing anaemia just with signs and symptoms is known by various differential diagnosis, like other high output states (thyrotoxicosis, aortic regurgitation), coronary artery disease, heart failure, especially in emergency situations. Also, there are evidences that anaemia contributes to cardiac diseases and death. For example in chronic kidney failure, anaemia is an independent risk factor for development of cardiovascular disease [24]. There is growing evidence that anaemia contributes to cardiac disease and death, independently as well as a co-morbid factor. Thus by picking up/ not missing anaemia by signs and symptoms and supported by ST depression, T wave changes, with/without associated QRS abnormalities (LVH) in ECG as seen in the present study, in any situation is advantageous, that wrong treatment can be avoided in emergency situations [20], dramatic clinical and electrocardiographic recovery can be achieved with anaemia correction [21] and better clinical outcomes can be achieved in conditions of anaemia as a co-morbid factor [25].
We have not considered the etiology of anaemia, duration of anaemia, bone marrow picture, and to confirm the cardiac changes we could not do echocardiography in the present study.
Conclusion
Thus, it can be concluded that diagnosing anaemia in critical care can be supported by ECG changes to avoid misdiagnosis and also as dramatic clinical and ECG recovery can be achieved with anaemia correction.
N = Number of patients, n= Number of patients in sub group, LVH = Left ventricular hypertrophy, STD = ST depression, Tw = T wave changes, HS = Highly significant, NS = Non significant