Infertility is a major reproductive health problem. Worldwide reports suggest that infertility affects an estimated 10% to 15% of couples. Nearly 50% of these are accountable to the male partner [1]. Various causative factors have been identified for male infertility including varicocele, obstruction of spermatic duct, erectile dysfunction and failure of ejaculation, various conditions causing imbalance in levels of sex hormones Testosterone, dihydrotestosterone, Follicle stimulating hormone (FSH), Leutinizing hormone (LH), Androgen receptor and last but not the least genetic defects. Environmental factors have also been considered in causing a decline in male reproductive health. In more than 60% of cases of infertility the origin of altered testicular function is unknown [2]. Genetic factors have been found to play a role in about 10% of male infertility. In about 15% cases of male infertility no organic cause is traceable (idiopathic infertility). Male fertility depends on successful spermatogenesis, in which a number of genes participate. So, it is evident that deletions or mutations in genes controlling spermatogenesis would result in infertility [3].
The male sex-determining region (SRY) has been mapped on the short arm of Y chromosome at Yp11. The critical genes involved in the sequential cascade of process of spermatogenesis are located on the long arm of the Y chromosome (Yq11). Researchers reported azoospermic individuals with microscopic deletions of distal euchromatic part of long arm of the Y-chromosome and on the basis of these findings in azoospermic men [4], they proposed existence of a spermatogenesis gene complex called “azoospermia factor” (AZF) on Yq. Yq part mainly divided in important AZFa, AZFb and AZFc regions [5]. Most of the deletions occur de novo and in confinement of one or more of these non-overlapping regions, AZFc being the most frequently deleted. AZFa, AZFb and AZFc are considered to be the most crucial region of Yq concerned with sperm production. Major candidate genes in AZFa region are USP9Y, DBY, UTY and TB4Y, in AZFb region there are EIF1AY, PRY, TTY2, RBMY genes and in region AZFc the genes are DAZ1, DAZ2, BPY2, PRY and CDY. USP9Y, RBMY and DAZ genes exist in multiple copies, of which only some are functional [6]. ‘Deleted in azoospermia gene-DAZ’ that exists in AZFc region, is expressed specifically in testis. DAZ is also the most frequently deleted site in non-obstructive severe or mild oligozoospermic infertile men [7,8]. Deletions in the AZFa and AZFb regions have been associated with azoospermia. The microdeletions in the AZF regions not only reflect as altered seminal parameters but correlate to varied testicular histological profile ranging from sertoli cell only syndrome (SCOS) to hypospermatogenesis [1].
According to global estimate about 10% cases of idiopathic azoospermia and oligozoospermia occur due to be deletions in AZF region. Therefore AZF deletions are amongst most common causes of spermatogenic failure in man identifiable by molecular genetics tool [9]. The aim of this study was to identify the microdeletions in AZFa, AZFb and AZFc regions of human Y chromosome by STS markers and to correlate the deletion-patterns with clinical phenotypic profile such as seminal parameters, hormonal status and testicular histology. Attempts to be made to find out any site specific microdeletions of Y chromosome in idiopathic azoospermic and oligozoospermic cases from central India population.
Materials and Methods
Selection of patients
We included 179 infertile men in present study, belonging to the age group of 25-45 years (32.18 + 4.61) as cases and 50 age matched fertile men as controls. Infertile men were referred to our human molecular division of cytogenetics lab from institutional reproductive biology unit. All the subjects enrolled as cases had primary infertility for more than one year. With a 3-days period of sexual abstinence, semen sample was obtained from each infertile subject. Complete semen analysis was performed including semen volume, pH, sperm concentration, motility and morphology. On the basis of seminal profile, infertile subjects were categorized as azoospermia (no sperm count), severe oligozoospermia (sperm count below 5 million per ml) and oligozoospermia (5-20 million sperm per ml) [10]. Pre structured questionnaire was completed by all the subjects. Among these subjects having no gonadal abnormalities, varicocele or a history of cryptorchidism, mumps, orchitis, diabetes or testicular injury were selected.
We also recorded the history of habits like alcoholism, smoking, tobacco chewing and made a note of any major illness in the family. In azoospermic men trans-rectal sonography was performed to known the status of ejaculatory duct, seminal vesicle abnormalities etc. The scrotal ultra-sonography was done to assess the testicular volume and abnormalities. The serum level of Follicle stimulating hormone (FSH) and Luteinizing hormone (LH) were recorded in each subject by radioimmunoassay (RIA). Nineteen (19) azoospermic and severe oligozoospermic infertile subjects were excluded from the study because of abnormal hormones profile of FSH, LH, Free Testosterone. In a few selective cases testicular biopsy was taken for study of histology. Informed consent forms were obtained from each infertile male before inclusion in study. The Institutional ethical committee had approved this study.
Chromosomal Analysis was done on phytohemagglutinin-M stimulated whole peripheral blood culture following standard protocol of our lab. Those infertile subjects with a normal 46,XY karyotype as shown by GTG banding were included in present study.
Sequence-tagged site (STS) PCR used for defining microdeletions
Human genomic DNAs were extracted from peripheral blood lymphocytes of 160 non-obstructive idiopathic infertile men, 50 normal healthy proven fertile subjects as positive controls and 5 normal women as negative controls using a phenol-chloroform Proteinase-K standard protocol [8,11].
Different STS primers were used for 18 AZF loci, four primers: sY746, sY86, sY84 & DFFRY for AZFa; 7 primers: sY113, sY118, sY127, RBMIY, XKRY, sY134, & sY143 for AZFb and 7 primers: sY153, sY148, sY157, sY255, sY254, sY158, sY160 for AZFc. All STSs were used in series of order and the localization of the sequences proposed by Vollarth 1992. The internal control was STS primer sY14 for sex determining region of the Y (SRY). 50-100 ng DNA samples were subjected to PCR amplification reactions with 1 X PCR assay buffer, 1.5 mmol/l MgCl2, 200 μmol/l deoxy-nucleotidetriphosphate (dNTPs), 1μmol/μl of each primer pair and 0.5 U Taq-DNA polymerase (Merck Biosciences, Bangalore, India). The reactions were performed in a Veriti 96 well thermal cycler (Applied Biosystem, CA, USA) for 30 cycles using standard protocol. Initial denaturation was done at 94°C for 3 min. & final extension at 72°C for 7 minutes in the last cycle. The subsequent denaturations were set at 94°C for 60 second, and extension at 72°C for 60 second and same for all the samples.
Different annealing temperatures that were used (56°C -60°C for 30 second- 50 second), depending on different types of STS markers. PCR product was analyzed on a 2 % agarose gel electrophoresis containing Ethidium bromide (0.5 mg/ml). In the event of detecting a microdeletion of a Y-specific sequence tagged sites (STSs) with a primer, the PCR assay was repeated thrice for confirmation.
Statistical Analysis
The clinical findings of infertile men are given as mean ± SD. All computations were carried out with the SPSS statistical analysis software. p<0.05 was considered significant.
Results
Chromosomal analysis was done on 160 idiopathic infertile men, among these 96 (60%) had oligozoospermia and 64 (40%) had azoospermia. However 3 subjects (P-54, P129 & P35) were 47,XXY Klinefelter’s Syndrome (KFS) and one was (p-35) 47,XYY male and no other structural chromosomal arrangement or Y chromosomal deletion was detected in GTG banding preparation. All infertile men were subjected for chromosomal analysis and Y deletion mapping simultaneously. However, Klinefelter’s syndrome and 47,XYY male were excluded from category of idiopathic infertile men. STS-PCR analysis was done in 156 cytogenetically normal infertile men and 50 fertile controls.
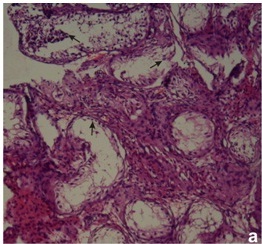
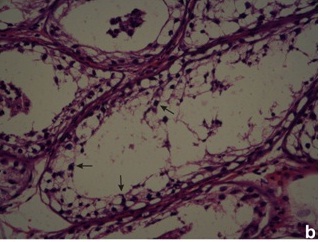
In PCR Yq microdeletion analysis, 13 infertile men (8.33%) with normal cytogenetic profile were detected with the presence of either one or more deleted STS loci. All deletions were recorded in interstitial region of Yq11 in the AZF locus of five oligozoospermic and eight azoospermic infertile men [Table/Fig-1]. No deletions were identified in any of the 50 fertile men. In normal fertile women, negative control, STS failed to amplify. The STS-PCR data of the 13 infertile subjects with deletion at Yq11 is presented in [Table/Fig-2]. Histopathology of testis biopsy in subjects P27 (azoospermia) and P132 (severe oligozoospermia) had shown the germ cell maturation arrest [Table/Fig-3a] with AZFc loci microdeletions and patient P103 (azoospermic) had germ cell development arrest at spermatid stage [Table/Fig-3b] with AZFc deletion of sY153, sY148, sY157 STS markers.
Gel illustration showing amplified products of sequence tagged sites (STS) markers corresponding AZFa, AZFb, AZFc regions
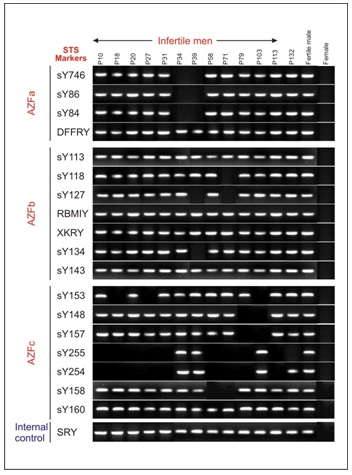
Findings of 13 infertile men with Yq deletions
| Patients |
|---|
| P10 | P18 | P20 | P27 | P31 | P34 | P39 | P58 | P71 | P79 | P103 | P113 | P132 |
|---|
| Left/Right testicular Volume (ml) | 10.6/12.1 | 9.32/7.4 | 10.31/6.19 | 12.3/7.52 | 11.6/7.93 | 10.8/12.43 | 10.71/11.5 | 6.47/8.61 | 9.30/8.42 | 13.7/10.5 | 12.5/11.91 | 8.37/6.73 | 11.48/9.51 |
| Sperm Concentrations (cell x 106/ml) | 0 | 2 | 2.5 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 3.5 | 0.1 |
| Serum FSH/LH (mIU/ml) | 9.33/ 8.29 | 12.36/ 6.81 | 10.05/ 4.66 | 15.1/ 7.92 | 12.7/ 8.2 | 18.34/ 9.62 | 12.54/ 7.16 | 10.39/ 6.72 | 10.8/ 7.48 | 14.6/ 9.2 | 12.4/ 6.91 | 15.47/ 7.21 | 10.72/ 6.25 |
| Testicular Histology | NP | NP | NP | Maturation Arrest | NP | NP | NP | NP | NP | NP | Development Arrest at spermatid stage | Maturation Arrest | |
| Karyotype | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY |
| Deleted AZF regions | c | c | c | c | c | a | a, b | c | b, c | c | c | c | c |
Mean +SD: FHS= 12.68+2.58 mIU/ml; LH= 7.41+ 1.28 mIU/ml; Right testicular vol.= 10.57 +1.90ml; Left testicular vol.= 9.28 +2.17 ml; AZF= Azoospermia Factor
(a): Photomicrographs showing primary spermatogenic arrest (arrows)
(b) absence of mature sperms in seminiferous tubules (arrows)
Andrological examinations
The mean volume of left and right testis were 9.28 ±2.17 ml and 10.57 ±1.90 ml respectively in 13 infertile males with microdeletions compared to 9.76 ±1.85 ml and 11.20 ±1.92 ml in 143 males without Y chromosome deletions. In males with deletions the values were slightly below as compared to those without deletions, but results were insignificant (t-test).
Hormones
The mean serum FSH and LH were 12.68 ±2.58 mIU/ml and 7.41 ±1.28mIU/ml respectively in 13 infertile men with Yq microdeletions compared with 11.39 ±1.83 mIU/ml and 7.26 ±1.41 mIU/ml in 143 infertile men without Yq microdeletions.
Discussion
In present study we assessed the prevalence of Y chromosome microdeletions in male infertility and made an attempt to find out the relationship between microdeletions and male infertility from central India population. Tiepolo & Zuffardi (1976) proposes the location of spermatogenesis controlling factors on nonfluorescent euchromatic portion of ‘q’ arm of Y chromosome. Comprehensive Y-chromosomal mapping studies comprising of large number of severely infertile men with identified phenotypes, revealed common deletion pattern in azoospermic males [12]. The frequently deleted region was located in AZFc. Y chromosome deletions mostly arose de novo and these deletions were the cause of spermatogenic failure observed in infertile subjects. DAZ genes cluster has frequently deleted and frequencies of deletions increase with the severity of spermatogenic defects in infertile subjects [13].
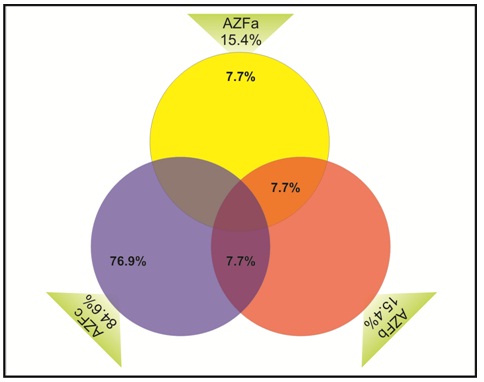
In the present study, thirteen infertile azoospermic & oligozoospermic men (8.33%) who were karyotypicaly normal showed microdeletions of Y chromosome spanning over the AZF a, AZFb, AZFc loci. Efforts have been made to determine the incidences of Yq microdeletions and to correlate the size and site of the Yq deletion with the infertile phenotype. Regionwise percentage of microdeletions are shown in [Table/Fig-4] and deletion mapping of the total 13 subjects of AZF regions demonstrated that the AZFc was having the highest number of microdeletions in 11(84.6%) infertile men and more commonly microdeletions were detected by STS primers sY254 & sY255 which cover the DAZ gene [Table/Fig-1]. Two infertile azoospermic males P34 and P39 were identified with AZFa and AZFa+b large deletions respectively, while subject P71 was found with isolated AZFb+c deletion.
Vendiagram shows percentage of microdeletions in AZF regions. The AZFa region was involved in 15.4%, of which AZFa alone was involved in 7.7% where as AZFa & AZFb were involved in 7.7%. AZFb region was also involved in 15.4% deletions. Deletion of AZFc found 84.6%, of which deletion of AZFb&c were 7.7% and deletion of AZFc alone was 76.9%
Frequency of Yq microdeletions observed in both oligozoospermic and azoospermic men is compared with the reports from different parts of world and other regions in India [8,14]. In south European population (Italy) the average frequency is around 15% while in north European populations (Germany, France, The Netherlands etc.) the frequency of Y chromosome deletion in infertile cases is low (1-4%) [1]. The data available from Australia, New Zealand, China, Japan and south East Asian countries the reported frequencies were around 10% [15]. However, the prevalence differs in a wide range, from 1% to 55%. Majority of the reported cases in Asian population deletions noted in AZFc region and occasionally in AZFa and AZFb regions [16]. The reported frequencies of microdeletions range from 2% to 10% in India. Deletion in AZFc region is more frequently observed as compared to AZFa and AZFb loci invariably in all studies [17]. Among these studies different loci of STS, including European Andrology Associations (EAA) recommended markers had been used for detection of Y chromosome microdeletion of infertile male population [9]. Thangaraj et al., found overall 8.5% deletions on AZF region in azoospermic men. Dada et al., had documented in 9.63% subjects showing deletion in AZF region in idiopathic infertile men [18]. Ambasudhan et al., showed around 5% deletions [19] and Mitra et al., reported around 5.29% deletions in oligozoospermic and azoospermic subjects [20]. Singh and Raman et al., studied microdeletion of Y chromosome. They stated AZFc to be the most frequently deleted region in azoospermic / oligozoospermic men [21]. These differences in deletion frequency among these reports from India may be due to selection bias of subjects and the choice of STS markers. The different geographical regions, environmental factor, occupational exposures and many ethnic populations have also been stated to be influencing deletion frequency of Y chromosome in infertile males. All these conditions might be necessary to determine the variation in deletion pattern and frequency in different ethnic and environmental influences worldwide populations [2,17].
The testicular volume, FSH and LH levels in AZF deleted males seem to vary in different studies. In present study, testicular volume did not differ significantly between infertile men with and without deletion. Similarly, insignificant differences in the mean of testicular volume were found in some other studies [21]. Serum FSH and LH level were recorded significantly changes between infertile men and normal fertile control subjects groups.
On correlating the deletion pattern with testicular histology the deletion of AZFa was found to be associated with complete absence of germ cells and presence of sertoli cells in the seminiferous tubules. The deletion of AZFb was associated with developmental arrest of germ cells at pachytene stage. The deletion of AZFc was variably associated with sertili-cell-only syndrome (SCOS), developmental arrest of germ cells at spermatid stage and maturation arrest. The genes at each locus act at a particular stage of germ cells differentiation and deletion of a particular AZF locus results in a characteristics phenotype [21,22]. Our study testicular biopsy was obtained in three infertile men, two with azoospermia one with severe oligozoospermia. Histopathological picture in all the three subjects comprised of developmental arrest of germ cells and maturation arrest [Table/Fig-1], [Table/Fig-3a], [Table/Fig-3b]. These subjects were having microdeletions in the AZFc region. So, our findings are in agreement that deletion of AZFc is associated with the developmental arrest of germ cells at spermatid stage and maturation arrest [1]. Further, identification of gene products and their biological actions is necessary to observe the influence of AZF gene deletions on formation of mature spermatozoa. Some studies have shown that genetic basis of infertility should be addressed after a vivid consideration of different genetic components like X chromosome mutation [23] and mitochondrial DNA mutations [24].
The finding of 8.33% incidences of microdeletions of Y chromosome confined to AZF a, b and c regions in azoospermic and severe oligozoospermic infertile subjects, supports the views that this specified regions of Y chromosome is essential for regulation of spermatogenesis. Deletions in the crucial AZF regions of Y chromosome are adversely affecting the seminal profile leading to infertility. The relevance of the present study was the higher percentage of deletions detected in AZFc region. These results suggest that genes in the Yq euchromatic segment may be strongly associated with testicular germline population. Idiopathic male infertility, a multifactorial disorder, is governed by environmental factors and genetic components. The etiology to male infertility may differ between ethnic populations. Therefore, it is very important to take ethnic-environmental-geographical axis into consideration in the genetic basis of male infertility. However, Y chromosome microdeletions cannot be predicted clinically, on the basis of semen analysis or by karyotyping. Our data will be useful for infertility clinics for genetic counselling and to adopt appropriate methods for assisted reproduction. Thus PCR based Y chromosome screening for microdeletions are the appropriate approach for diagnosis, management and counselling of the infertile men.
Mean +SD: FHS= 12.68+2.58 mIU/ml; LH= 7.41+ 1.28 mIU/ml; Right testicular vol.= 10.57 +1.90ml; Left testicular vol.= 9.28 +2.17 ml; AZF= Azoospermia Factor