Role of S-100 Immunostaining in Demonstration of Nerve Changes and Quantification of Dendritic Cells in Leprosy
Anand Mohanraj1, Sowmya Srinivasan2
1 Postgraduate, Department of Pathology, Sri Manakula Vinayagar Medical College and Hospital, Kalitheerthalkuppam, Puducherry, India.
2 Professor and Head, Department of Pathology, Sri Manakula Vinayagar Medical College and Hospital, Kalitheerthalkuppam, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sowmya Srinivasan, Sri Manakula Vinayagar Medical College and Hospital, Puducherry-605107, India.
Phone: 9003487100,
E-mail: drssowmya@hotmail.com
Background: A definitive diagnosis of leprosy is based on a demonstration of either acid-fast bacilli or nerve elements within the granulomas. On routine hematoxylin and eosin stains, the nerve fibers are not easily identifiable. In this study, S-100 immunostain is used to highlight the nerve elements and to demonstrate and compare the nerve changes in spectrum of leprosy including reactions.
Aim: To demonstrate the nerve changes in spectrum of leprosy using S-100 immunostaining so as to categorize them for the purpose of early diagnosis and treatment. We also want to demonstrate and quantify the dendritic cells in lepromatous spectrum of leprosy using S-100 immunostain.
Materials and Methods: Twenty consecutive skin biopsy specimens from patients with histopathological diagnosis of leprosy in the year 2012 were studied. Of these 20 cases, 13 were Borderline Tuberculoid, 1 was of indeterminate leprosy, 1 Borderline Lepromatous, 2 cases of Lepromatous Leprosy, 1 case of Type 1 reac-tion and 2 cases of Type 2 reaction. Stains used were Hematoxylin and Eosin stain for the histopathological diagnosis, Fites stain for Bacillary index and S-100 immunoperoxidase staining for nerve changes. 5 cases of granulomatous dermatosis of skin other than leprosy (5 cases of lupus vulgaris) were included as controls.
Results: On Hematoxylin and Eosin staining, the nerve fibers showed vertical orientation in relation to epidermis in Borderline Tuberculoid leprosy. In addition, the nerve fibers showed rounded contour in Tuberculoid leprosy. The entire spectrum of leprosy showed evidence of nerve damage in S-100 immunostaining which was categorized in 4 patterns 1. Absent, 2. Fragmented, 3. Discontinuous and 4. Intact. The majority of Borderline Tuberculoid leprosy cases showed absent pattern of nerve damage. Dendritic cells were also positive for S-100 immunostaining with granular positivity in Borderline Tuberculoid Leprosy cases and membranous positivity in Lepromatous spectrum.
Conclusion: Nerve damage is seen across the entire spectrum of leprosy and the early identification of this nerve damage using S-100 immunostaining, helps to differentiate between Lepromatous and Tuberculoid leprosy, especially in the borderline and indeterminate forms.
Leprosy, Nerve fibers, Dendritic cells, S-100 immunostaining
Introduction
Leprosy is a chronic disease caused by Mycobacterium leprae and affects predominantly the peripheral nervous system and skin. Though the Ridley-Jopling classification is widely accepted, its histological component refers only to skin, with the presumption that there may be no significant difference in the classification of skin and neural histology [1]. However discrepancies between nerve and skin lesions were noted in the form of increased bacilli and a lower lepromatous histological grading in nerves than in skin [2,3]. Nerve damage and sensory impairment are central to the pathogenesis of leprosy. On routine Hematoxylin and Eosin stain, the nerve fibers do not stand out well from the background [4,5]. This is of significance, especially in Tuberculoid and Indeterminate forms, where it is not possible to demonstrate Mycobacterium leprae bacilli using Fites stain [6]. Another difficulty arises in differentiating Tuberculoid forms of leprosy from other granulomatous diseases [4,6]. In an endemic area the diagnosis of leprosy should always be considered, when there is a compatible clinical presentation and histologic picture even in the absence of demonstrable Mycobacterium leprae bacilli [6]. Lesions mimicking Tuberculoid leprosy include granulomatous secondary syphilis, post Kala-Azar Dermal Leishmaniasis, Sarcoidosis, Granuloma Annulare, Lupus Vulgaris [6,5].
Thomas MM et al., have found that S-100 is a sensitive and reliable marker of nerve damage [4]. The positive staining of Schwann cells allows easy recognition of intact dermal nerve twigs or their fragments [4,6]. The difficulty in recognizing small nerve twigs on routine Hematoxylin and Eosin sections has led to the use of S-100 stain as an aid to the histological differentiation of granulomatous skin inflammations [4,6]. The finding of nerve twigs within a granuloma should suggest a diagnosis of leprosy [6]. According to Thomas MM et al., and Gupta SK et al., there are four patterns of nerve damage demonstrable on S-100 immunostaining, namely 1. Infiltrated 2. Fragmented 3. Absent 4. Intact [4,7]. According to Gupta SK et al., the patterns 1,2,3 had sensitivity, specificity, positive and negative predictive value of 100% in diagnosing Tuberculoid Leprosy (Tuberculoid and Borderline Tuberculoid) [7]. Dendritic cells have an important role in the pathogenesis of leprosy by contributing to the immune response in leprosy [8]. Dendritic cells are recruited in large numbers in both Tuberculoid and Lepromatous leprosy, but the transformation of dendritic cells to Langerhans cells is impaired in Lepromatous leprosy [8]. S-100 stain can be used for demonstrating and quantifying dendritic cells [8].
Aim
This study was carried out for the purpose of demonstrating nerve changes in the entire spectrum of leprosy using S-100 immunoperoxidase staining, to show the various patterns of nerve damage, along with dendritic cell morphology.
Materials and Methods
Skin biopsies of patients diagnosed as leprosy in the year 2012 in the Pathology department of SMVMCH were taken for the study. These skin biopsies were examined histologically using Hematoxylin and leprosy Eosin staining for diagnosis of the spectrum of leprosy, Fites stain for Bacillary index and S-100 immunostaining for nerve changes. Of these 20 cases, there were 13 cases of Borderline Tuberculoid Leprosy 1 was of indetermi-nate leprosy, 1 Borderline Lepromatous, 2 cases of Lep-romatous Leprosy, 1 case of Type 1 reaction and 2 cases of Type 2 reaction. The histopathological diagnosis and Bacillary Index (BI) were done according to the Ridley-Jopling classification. Five cases of non lepromatous granulomatous dermatosis of skin were used as control and subjected to S-100 immunostaining.
Observation and Results [
Table/Fig-1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11and
12]
| Score | Granulomas | Lymphocytes | Histiocytes | Dendritic Cells |
|---|
| 0 | Nil | Nil | Nil | Nil |
| 1 | Solitary | Scanty | Occasional | Occasional |
| 2 | Perivascular, Periadnexal | Around granulomas | Few around granulomas | Few |
| 3 | Diffuse | Diffuse | Multiple | Dense |
Epidermal, dermal changes & bacillary index in the spectrum of leprosy
| Morphology | BT | Indeterminate | BL | LL | Type 1 reaction | Type 2 reaction |
| Epidermal Changes | Nil | Nil | Nil | Flattening | Nil | Flattening |
| Lymphocytes | 2 | 1 | 2 | 0 | 0 | 1 |
| Histiocytes | 0 to1 | 0 | 1 | 3 | 2 | 3 |
| Granulomas | 2 | 0 | 0 | 0 | 2 | 0 |
| Bacillary Index | Nil | 1+ | 2+ | 5+ | Nil | 5+ |
Nerve changes in the spectrum of leprosy on H&E stain
| Nerve Changes | BT | Indeter-minate | BL | LL | Type 1 | Type 2 |
| Location | Within Granu-lomas | Non-specific | Non-specific | Nonspecific | Within Granulomas | Mixed |
| Orientation | Vertical | Vertical | Vertical | Vertical | Mixed | Mixed |
| Morphology | Rounded outline | Rounded outline | Sharp contour | Sharp contour | Mixed | Mixed |
| Nerve Destruction | + | + | + | + | + & intact | + |
Neuronal changes and dendritic cells on S-100 immunostain
| Neuronal Changes | BT | Indeterminate | BL | LL | Type 1 | Type 2 |
| S-100 Staining pattern | Absent to fragmented | Absent to fragmented | Absent | Absent | Intact to Disco- ntinuous | Absent to fragm- ented |
| Dendritic cells | Absent to occasional granular | Negative | 2+, Mem- branous | 3+, Mem- branous | +, Granular | 3+, Mem- branous |
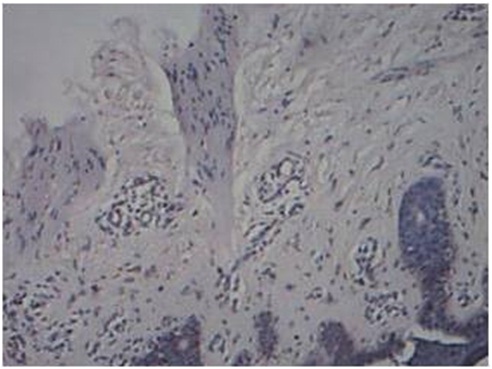
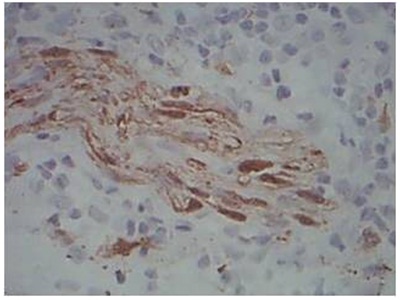
Nerve bundles vertically oriented in relation to epidermis in BT Leprosy, H&E, 20x
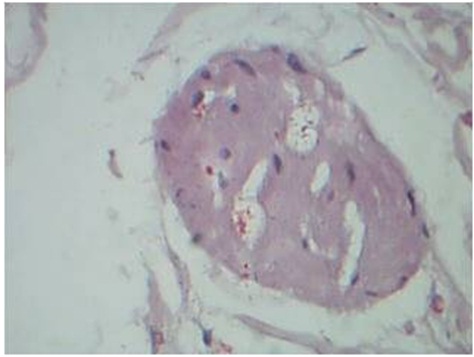
Bacilli within nerve in BL Leprosy, Fites stain, 40 x
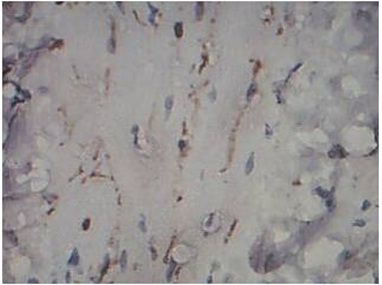
Absent pattern of nerve change in BT Leprosy, S-100 immunostain, 40x

Fragmented pattern of nerve change in BT Leprosy, S-100 immunostain, 40x
Discontinuous pattern of nerve change in Type 1 reaction, S-100 immunostain, 40x
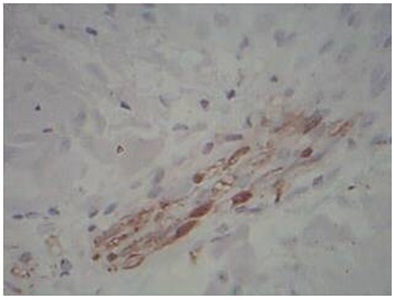
Intact pattern of nerve change in Type 1 reaction, S-100 immunostain, 40x
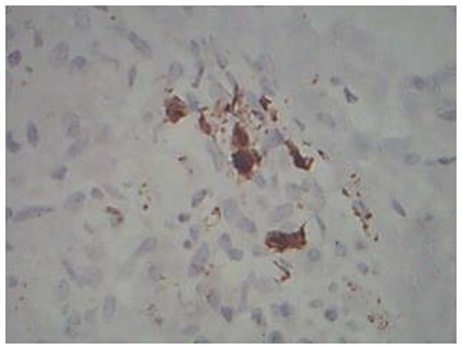
Granular staining of Dendritic cells in BT Leprosy, S-100 immunostain, 40x
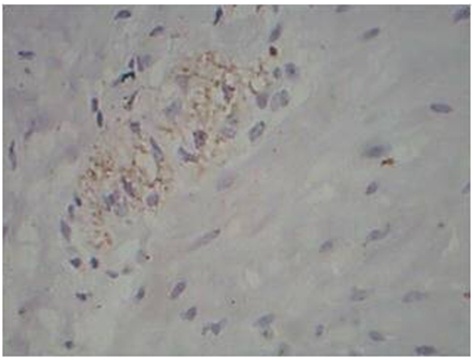
Membranous staining of Dendritic cells in LL leprosy, S-100 immunostain, 40x
Epidermal Changes: Thirteen cases of Borderline Tuberculoid Leprosy showed no changes, with one case of Lepromatous Leprosy and Type 2 reaction showing flattening of epidermis.
Contour of Nerve: Tuberculoid Leprosy showed predominantly a rounded contour of nerve. Mixed morphology of nerves was observed in both patterns of reaction. The entire spectrum of leprosy showed evidence of nerve destruction. In the 5 cases of control (Lupus vulgaris) the nerve fibers had a sharper outline.
Orientation of Nerve: Borderline Tuberculoid and lepromatous leprosy showed vertical orientation of nerve fibers in relation to epidermis. Types 1 and 2 reactions showed mixed orientation of nerve fibers. The 5 cases of Lupus vulgaris also showed a vertical orientation of nerve fibers.
Patterns of Nerve Destruction in S-100: Four different patterns of nerve destruction were observed in S-100 immunostaining. They are 1. Absent 2. Intact 3. Fragmented 4. Discontinuous. Ten out of thirteen cases of Borderline Tuberculoid leprosy showed absent pattern, with one case showing both absent and fragmented pattern and two cases showing only fragmented pattern. Indeterminate and Lepromatous leprosy showed absent pattern of nerve staining using S-100. One case of Type 1 reaction showed both intact and discontinuous pattern of staining. One case of Type 2 reaction showed absent pattern of staining and the other showed fragmented pattern of staining. All cases of control showed intact pattern of nerve fibers staining using S-100 immunostain.
Dendritic Cells on S-100: Lepromatous spectrum, and type 2 reaction showed increased number of dendritic cells on S-100 immunostaining. The pattern of staining was absent to minimal granular intracytoplasmic positivity in Tuberculoid spectrum. In Lepromatous spectrum, there was intracytoplasmic and membranous positivity.
Discussion
Leprosy is diagnosed based on morphology using Hematoxylin & Eosin stain and bacillary index with Fites stain. However the nerve changes which occur as an early manifestation and which is an indicator of deformities cannot be visualized. The diagnosis of Lepromatous leprosy is fairly straight forward with demonstration of bacilli, but the diagnosis of Tuberculoid leprosy, along with the indeterminate forms is extremely difficult on routine Hematoxylin & Eosin staining. S-100 immunostaining is superior to Hematoxylin and Eosin staining in identifying the destruction of dermal nerves in leprosy [5]. Fleuri and Bacchi in their study found that 8 out of 9 biopsies with a clinical diagnosis of Tuberculoid leprosy, but with no histological or bacteriological evidence of the same, showed cutaneous nerve alteration by S-100 staining [9]. With the World Health Organization’s goal of reducing the prevalence of leprosy to 1 per 10,000 population by the year 2000 [10], the expertise to diagnose leprosy may be expected to decrease and S-100 immunoperoxidase staining could prove to be an effective aid in the diagnosis and study of patterns of cutaneous nerve involvement especially in the Tuberculoid forms of leprosy [5].
In Borderline Lepromatous and Lepromatous leprosy, the large numbers of M. leprae staining positively with the S- 100 stain may interfere with the search for nerve branches. However lepra cells are clearly different from the fibrillary structure of nerve branches [6]. In the present study, on Hematoxylin and Eosin staining, the entire spectrum of leprosy cases showed features of nerve destruction. The cases of Borderline Tuberculoid, Lepromatous, Indeterminate and Borderline lepromatous leprosy showed vertical orientation of nerve fibers in relation to epidermis. The cases of reaction showed mixed orientation in relation to epidermis. Rounded contour of nerve twigs were noted in Tuberculoid leprosy cases as reported by Singh N et al., [6].
Four different patterns of nerve damage were observed: infiltrated, fragmented, absent, and intact [4]. The majority of cases of Borderline Tuberculoid leprosy and Lepromatous leprosy spectrum, showed absent pattern of staining of nerve twigs on S-100 immunostaining which indicates nerve destruction. These findings were in contrast to the findings seen in the study by Thomas MM et al., where the predominant pattern of nerve damage seen was fragmented pattern of nerve damage followed by absent pattern of nerve damage [4].
The cases of Lepromatous spectrum and Type 2 reaction showed increased number of dendritic cells, which showed membranous positivity of staining on S-100 immunostaining. Dendritic cells were either absent or only a few in number in Borderline Tuberculoid, Indeterminate leprosy, Type 1 reaction with granular positivity for S-100 immunostaining. These findings were consistent with studies by Azadeh and Dabiri which showed that the number of dendritic cells were significantly increased in Lepromatous spectrum, but the maturation / migration to epidermis is impaired in Lepromatous Leprosy [8]. We were able to supplement this concept in our study by a comparison of Bacillary index and dendritic cell density by S-100 immunostain. In both Borderline lepromatous leprosy & Lepromatous leprosy, the bacillary index was 3+ and 5+ respectively and the dendritic cellularity was also markedly increased ranging from 2+ to 3+, along with a membranous pattern of staining. Similar picture was also seen in Type 2 reaction. These findings suggest that there is increased recruitment of dendritic cells in response to increased bacillary index in Lepromatous spectrum, but the maturation/migration to the epidermis is impaired, as Suggested by Azadeh, Dabiri and Abulafia J and Vignale RA, [8,11].
Summary
A confirmatory diagnosis of leprosy can be made on the basis of finding active destruction of cutaneous nerves by granulomatous inflammation in a skin biopsy [5]. Immunoperoxidase staining for S-100 protein which is a marker for Schwann cells [12], was used to delineate nerves in lesional skin biopsies of 20 cases of leprosy, extending across the entire spectrum. In Tuberculoid Spectrum of Leprosy, there is vertical orientation of nerve fibers in relation to epidermis with rounded contour of nerve. Entire Spectrum of leprosy showed nerve destruction [13]. The majority of cases of tuberculoid leprosy showed absent pattern of nerve staining using S-100. In all cases of Borderline Tuberculoid leprosy, there was granular positivity of dendritic cells using S-100. The lepromatous spectrum differed by having increased dendritic cells with membranous S-100 positivity. This indicates that S-100 immunostaining is useful in leprosy diagnosis not only by nerve staining patterns, but it also helps to differentiate the spectrum by highlighting the changes in dendritic cells [14]. The significance of this study lies in the application of S-100 to demonstrate nerve damage, for an earlier diagnosis [15]. An earlier diagnosis helps in reducing morbidity, drug resistance and achievement of elimination rates. Another important facet of this study is to arrive at an accurate diagnosis of Tuberculoid spectrum of leprosy, especially the borderline and indeterminate forms and to differentiate from other granulomatous diseases. There was also a causal relationship between Bacillary index and dendritic cell population in cases of Lepromatous spectrum.
[1]. Ridley DS, Jopling WH, Classification of leprosy according to immunity. A five-group systemInt J Lepr Other Mycobact Dis 1966 Jul-Sep 34(3):255-73. [Google Scholar]
[2]. Srinivasan H Rao KS, Iyer CGS, Discrepancies in histopathological features of leprosy lesions in the skin and peripheral nerveLepr in India 1982 54(2):275-82. [Google Scholar]
[3]. Ridley DS, Ridley MJ, Classification of nerves is modified by delayed recognition of Mycobacterium lepraeInt J Lepr 1986 54(4):596-605. [Google Scholar]
[4]. Thomas MM, Jacob M, Chandi SM, George S, Pulimood S, Jeyaseelan L, Job CK, Role of S-100 staining in differentiating leprosy from other granulomatous diseases of the skinInt J Lepr Other Mycobact Dis 1999 Mar 67(1):1-5. [Google Scholar]
[5]. Khan AR, S-100 protein in the diagnosis of tuberculoid/borderline tuberculoid leprosyAnn Saudi Med 1998 Jul-Aug 18(4):305-7. [Google Scholar]
[6]. Singh N, Arora VK, Ramam M, Tickoo SK, Bhatia A, An evaluation of S-100 stain in the histological diagnosis of Tuberculoid leprosy and other granulomatous dermatosesInt J Lepr Other Mycobact Dis 1994 Jun 62(2):263-7. [Google Scholar]
[7]. Gupta SK, Nigam S, Mandal AK, Kumar V, S-100 as a useful auxiliary diagnostic aid in tuberculoid leprosyJ Cutan Pathol 2006 Jul 33(7):482-6. [Google Scholar]
[8]. Azadeh B, Dabiri S, Langerhans cells in skin lesions of leprosyIJMS 2004 Jun Vol 29(No (2)) [Google Scholar]
[9]. Fleuri RN, Bacchi CE, S-100 protein and immunoperoxidase technique as an aid in the histopathologic diagnosis of leprosyInt. J. Lepr 1987 55:338-44. [Google Scholar]
[10]. World Health OrganizationA Guide to Leprosy Control 1998 2nd ednGenevaWorld Health Organization [Google Scholar]
[11]. Abulafia J, Vignale RA, Leprosy: pathogenesis updatedInt J Dermatol 1999 38:321-34. [Google Scholar]
[12]. Job CK, Drain V, Deming AT, Hastings RC, Gerber MA, Role of S-100 protein as a marker for Schwann cells in the diagnosis of tuberculoid leprosyInt J Lepr Other Mycobact Dis 1990 58(2):392-93. [Google Scholar]
[13]. Job CK, Nerve damage in leprosyInt J Lepr 1989 57:532-39. [Google Scholar]
[14]. Camargo LH, Caldas ML, Neira M, Sarmiento L, Image analysis to quantify S100-positive dendritic cells in leprosy-affected skinBiomedica 2003 23(2):131-33. [Google Scholar]
[15]. Kahn HJ, Marks A, Thom H, Baumai R, Role of antibody to S-100 protein in diagnostic pathologyAm. J. Clin. Pathol 1983 79:341-47. [Google Scholar]