Sertoli-Leydig Cell Tumor of Ovary- A Diagnostic Dilemma
Rohini Dhanya C.S1, Somanath Padhi2, Renu G’Boy Varghese3
1Tutor, Department of Pathology,Dr. B.R. Ambedkar Medical College and Hospital, Bangalore, India.Ex-Senior Resident,Department of Pathology,Pondicherry Institute of Medical Sciences, Ganapathychettykulam, Kalapet, Puducherry, India.
2Assistant Professor, Department of Pathology,Pondicherry Institute of Medical Sciences, Ganapathychettykulam, Kalapet, Puducherry, India.
3Professor and HOD, Department of Pathology,Pondicherry Institute of Medical Sciences, Ganapathychettykulam, Kalapet, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rohini Dhanya C.S., # 402, Rathna Nest, 1st Cross, Obamma Lane, S.R. Layout, Wind Tunnel Road, Murugeshpalya, Bangalore – 560017, India.
Phone: +91-9880040734,
E-mail: dr.rohinidhanya@gmail.com
Sertoli Leydig Cell Tumours (SLCTs) are rare, unilateral, sex cord stromal tumours of ovary, which constitute less than 1% of all the ovarian neoplasms. These tumours can be functionally diverse and they may have heterologous elements. We aim to report a case of a 25-year- old woman who presented with suprapubic pain of 5 days duration, a unilateral adnexal mass, hypertestosteronism without virilization. Intraoperative frozen section of the unilateral salpingo-oophorectomy specimen was suggestive of granulosa cell tumour. Histopathological examination, supplemented with alpha-inhibin immunohistochemistry, was diagnostic of Meyer’s type II SLCT. Clinical presentation, pathology and the diagnostic pitfalls in the present case have been presented with a brief review of literature.
Sertoli-Leydig cell tumour, Ovary, Alfa-Inhibin, Frozen section
Case Report
A 25-year-old married woman, mother of a single child, presented with abdominal pain of 5 days duration. She had a spontaneous abortion 8 months back, following which she had experienced irregular periods. Clinical examination revealed a moderately built female with a suprapubic mass of 16 weeks gestation size, in the right iliac fossa. Vaginal examination revealed a firm, mobile, non-tender, cystic mass in the right adnexa. No features of virilization were seen. Routine laboratory evaluation showed a microcytic hypochromic red cell morphology and low haemoglobin level of 90 g/L (reference; 120-140 g/L). Abdominal ultrasonography revealed a 10 x 8 cms sized, complex, solid to cystic mass in right ovary, which was suggestive of a dermoid. Hypertestosteronaemia of 41.7 ng/mL (reference; less than 2.2 ng/mL) and sex hormone binding protein (SHBP) of 142 nmol/L (reference; 15 to 120 nmol/L) was present. Based on these findings, a provisional diagnosis of an androgen producing ovarian tumour was made.
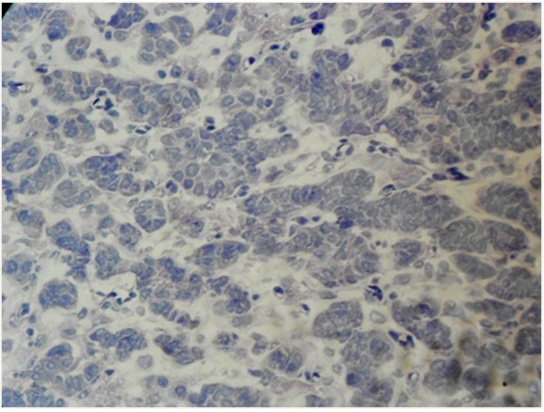
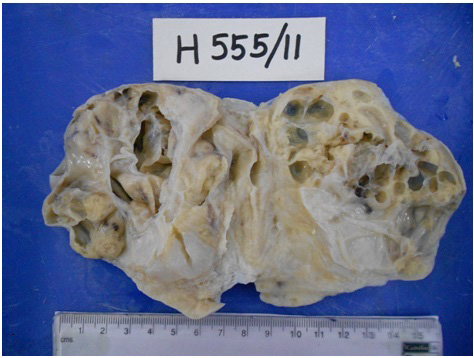
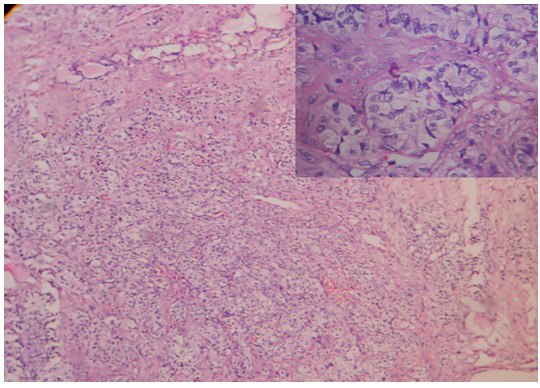
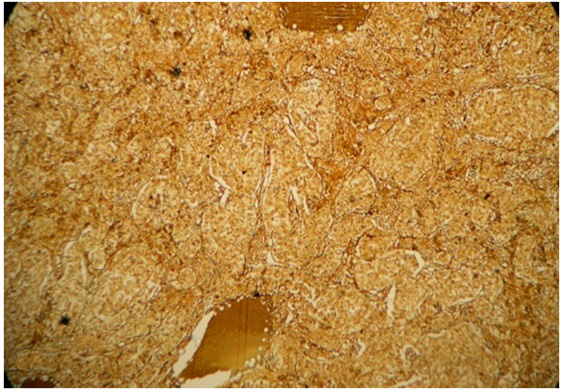
Per-operative frozen section of the unilateral salpingo-oophorectomy specimen, supplemented with Oil Red O staining, revealed a tumour with tiny cystic spaces which were lined by cuboidal cells and filled with eosinophilic material, which was suggestive of Call- Exner bodies of Granulosa Cell Tumour (GCT) [Table/Fig-1]. Gross evaluation revealed a 9.5x 8 x 1.5 cm solid-cystic ovarian mass with foci of yellowish areas, smooth multilobulated surface, and intact capsule [Table/Fig-2]. Paraffin embedded and haematoxylin-eosin stained tissue sections revealed Sertoli- like cells which were arranged in cords, nests and tubules in a markedly oedematous stroma. These cells were polygonal, with moderate cytoplasm, round to oval nuclei and uniform granular chromatin. Numerous sheets of polygonal cells with abundant granular eosinophilic cytoplasm (Leydig cells) were seen intervening between the Sertoli tubules. Heterologous elements were not seen [Table/Fig-3]. There was no breach of the capsule. Alfa-Inhibin immunohistochemistry done on deparaffinized tissue sections demonstrated strong cytoplasmic positivity in both Sertoli cells and Leydig cells [Table/Fig-4]. Peritoneal wash cytology was negative for malignancy. Hence, a diagnosis of intermediate (Meyer’s Type II) SLCT of the right ovary was made. On follow-up, there was no evidence of local recurrences or distant metastasis post surgery, and repeat testosterone levels were within normal reference range.
Cryostat tissue sections from the right ovarian tumor highlighting Call-Exner bodies like areas suggestive of a granulosa cell tumor (H&E,x400)
Gross photograph of right sided ovarian tumor specimen showing the solid-cystic component and focal yellowish areas
Formalin fixed paraffin embedded tissue sections from the right ovarian tumor showing Sertoli cells arranged in trabeculae, tubules, and cords along with islands of Leydig cells (H&E,x 100 (low power), x400(inset)
Alfa-inhibin immunohistochemical staining showing strong cytoplasmic positivity in the lesional cells (Peroxidase-antiperoxidase method,x200)
Discussion
Sertoli -Leydig Cell Tumours (SLCTs) of the ovary account for 1% of sex cord stromal tumours of ovary [1]. These tumours are rare in young adults [2,3]. Earlier, these tumours had terminologies like arrhenoblastomas and androblastomas [3]. The interesting fact is that the Sertoli cells and not the Leydig cells, form the neoplastic component of these tumours [1].
The review of literature regarding the clinical presentation, gross and microscopic pathology, management, and outcome of ovarian SLCTs, both in pregnant and non pregnant patients, have been presented in[Table/Fig-5]. Oliva et al., [4] reported a wide age distribution among 54 patients, of which 6 were associated with Peutz-Jeghers syndrome; and 18 of 22 were positive for α- inhibin. One case worsened with splenic metastasis. Young et al., [5] noted unilateral SLCTs in all 13 young pregnant women and recurrences were seen among ruptured ones. Some studies [1-3,7] have reported cases which presented with features of virilization and hypertestoteronaemia, that resolved in some women or persisted following surgery. A rare case of bilateral SLCT with peritoneal implants was highlighted by Alam et al.,[2]. Heterologous components comprising surface epithelium with serous and mucinous differentiations, mucinous epithelium of gastro-intestinal type were described by others [1,3,7-9]. In comparison, the present case had abdominal pain and swelling for 8 months following an abortion and hypertestoteronaemia without virilization. We presume that the metabolic capability of placenta as well as modulating effect of pregnancy may be the possible explanations for this clinicobiochemical discrepancy seen in our case [9].
On frozen sections, the presence of microcysts, microfollicle like structures resembling those seen in Call-Exner bodies, trabecular pattern, and nuclear grooves were suggestive of a granulosa cell tumour [1]. The trabecular, pseudopapillary pattern in some foci led to confusion with carcinoid tumour, papillary serous surface epithelial tumour. Ovarian SLCTs can have diverse histomorphological patterns like cords, trabeculae, tubules, diffuse sheets, pseudo papillary fronds and spindled, pseudo-alveolar, lipid rich forms, or retiforms, etc [1,2]. These diverse patterns, along with frozen section artefacts, may pose diagnostic challenges, especially during intra-operative consultations [8,9]. These tumours, when they are present during pregnancies, cause diagnostic dilemmas as well as difficulties in their management. However, strong cytoplasmic positivity for α-inhibin IHC in the lesional cells, seen in our case, supplemented with reticulin staining, was consistent with the diagnosis of a SLCT, as well as on ruling out other differentials (negativity for chromogranin and cytokeratin) [1,4]. Unilateral salpingo-oophorectomy was the treatment of choice without recurrences at 4 months post surgery.
To summarize, we have presented a case of androgen secreting ovarian SLCT with atypical clinical manifestations. In view of the rarity and the diversity of histological manifestations, the diagnosis may be difficult; especially in frozen section set-up. However, correlation with special stains and IHC are quite useful in delineating the true nature of the disease process.
Ovarian Sertoli-Leydig cell tumors in pregnant and non pregnant female patients
| Authors, Ref., year | No.of cases | Age (yreas) (mean) | Presentation, hormonal manifestation (n, %) | Site | Gross | Pathology (n, %) | IHC | Management | Outcome |
| Oliva et al., [4] | 54 | 2-76 (30) | PZ syndrome (6, 11%) | Most U/L | 8-30 cms, solid, yellowish areas | Tubular (most common), cords/trabeculae (28, 51%),diffuse (21, 38%), pseudopapillary (4, 7%), retiform (3, 5%), alveolar (3, 5%), spindled (3, 5%), lipid-rich (6, 11%) | Inhibin (18/22 +, 81%), Calretinin (10/20+, 50%),CD99 (19/22 +, 86%),vimentin (17/ 18+, 94%), SMA (4/18 +, 22%), NSE (8/16 +, 50%), S-100(2/20+, 10%), EMA & chromogranin- negative (100%) | U/L or B/L SO | NER 1 case of splenic metastasis |
| Young et al. [5] | 13 | Young pregnant women | Incidental, obstructed labour, rupture, androgenic (2%) during pregnancy or in postpartum | U/L | 3-22 cms (15), solid –cystic (7), solid (6), yellow areas (7) | Meyer’s type II (10/13), sheets of Leydig cells (8/13), necrosis & haemorrhage(8/13), intercellular edema +, heterologous elements-gastrointestinal type (2/13) | ND | O, Intact capsule (7), p/o rupture (5), I/o rupture (1) | Recurrences in ruptured cases (70%) |
| Alam et al., [2] | 1 | 17 years | Irregular menses, abdominal lump, no virilization. | B/L | Rt; 25x23x8 cms, Lt-10x 8x3 cms, solid-cystic, focal yellow areas, intact capsule | Frozen study done. Peritoneal implant+, retiform pattern, mitosis >5/10 HPF, Meyer’s type II. | ND | B/L SO | On follow up |
| Choudhary et al., [6] | 1 | 18½ years, married | Sudden onset of virilization, massive ascites | B/L | Ruptured capsule | Frozen study done. B/L (malignant) | ND | TAH + B/L SO + Om, debulking CT | NER, NEM. Voice change & hirsuitism persists. |
| Chakrabarti et al., [3] | 1 | 26 years | Virilization +, secondary amennorhea- 2 years. Serum testosterone- 5.2 pg/ml, reference= 0.7-3.6 pg/ml) | U/L | 13x10 cms, solid–cystic, intact capsule, contain mucinous material | Cords, trabeculae of SC, clumps of LC, heterologous elements-surface epithelium of serous & mucinous differentiation | - | U/L SO + CT- BEP regimen | Symptoms free at 6 months, on follow- |
| Tandon R et al., [7] | 1 | 19 years | Virilization +, amenorrhea (7 months), serum testosterone- 2ng/ml (0.2-1.2ng/ml) | U/L | 10x7X2 cms, gray white, solid–cystic, clear fluid, yellowish areas, intact capsule | Cords, nests, trabeculae of SC, interspersed LC, heterologous elements- mucinous epithelium of gastrointestinal type | - | U/L SO, CT-BEP regimen | Resolution of virilization symptoms |
| Present case | 1 | 25 years | H/o abortion 8 months back, irregular menses, abdominal pain & lump, virilization absent, serum testosterone-41.7 ng/ml (ref. <2.2ng/ml) | U/L | 10X9 cms, solid–cystic, clear fluid, yellowish areas,intact capsule | Frozen study; s/o GCT. Cords, tubules of SC, sheets of LC. Heterologous elements absent | α-Inhibin cytoplasm positivity | U/L SO | Testosterone levels back to normal range at 4 months of follow up |
α-Inhibin, alpha-inhibin; B/L, bilateral; BEP, Bleomycin, Etoposide and Cisplatin; CT, chemotherapy; EMA, epithelial membrane antigen; GCT, granulosa cell tumor; IHC, immunohistochemistry; i/o, intra-operative; LC, Leydig cells; NSE, neuron specific enolase; ND, not described; NER, no evidence of recurrence; NEM, no evidence of metastasis; O, Oophorectomy; Om, omentectomy; PJ, Peutz - Jeghers syndrome; p/o, pre-operative; SC, Sertoli cells; s/o, suggestive of; SMA, smooth muscle actin; SO, salpingo-oophorectomy; TAH, total abdominal hysterectomy; U/L, unilateral
α-Inhibin, alpha-inhibin; B/L, bilateral; BEP, Bleomycin, Etoposide and Cisplatin; CT, chemotherapy; EMA, epithelial membrane antigen; GCT, granulosa cell tumor; IHC, immunohistochemistry; i/o, intra-operative; LC, Leydig cells; NSE, neuron specific enolase; ND, not described; NER, no evidence of recurrence; NEM, no evidence of metastasis; O, Oophorectomy; Om, omentectomy; PJ, Peutz - Jeghers syndrome; p/o, pre-operative; SC, Sertoli cells; s/o, suggestive of; SMA, smooth muscle actin; SO, salpingo-oophorectomy; TAH, total abdominal hysterectomy; U/L, unilateral
[1]. M Nouriani, C Felix, L Dubeau, Histogenesis and histopathological characteristics of Sertoli-Leydig cell tumors.CME Journal of Gynaecologic Oncology 2002 7:114-20. [Google Scholar]
[2]. K Alam, V Maheshwari, S Rashid, S Bhargava, Bilateral Sertoli- Leydig cell tumoura rare case reportIndian J Pathol Microbiol 2009 52(1):97-99. [Google Scholar]
[3]. I Chakrabarti, A De, M Gangopadhyay, P Bera, Sertoli-Leydig cell tumour of ovary with heterologous elements—A case reportThe Internet Journal of Gynaecology and Obstetrics™.:1528-8439. [Google Scholar]
[4]. E Oliva, T Alvarez, RH Young, Sertoli cell tumors of the ovary: a clinicopathological and immunohistochemical study of 54 casesAm J Surg Pathol. 2005 29(2):143-56. [Google Scholar]
[5]. RH Young, AG Dudley, RE Scully, Granulosa cell, sertoli leydig cell and unclassified sex cord-stromal tumors associated with pregnancy - A clinicopathological analysis of 36 cases,Gynaecol Oncol 1984 18(2):181-205. [Google Scholar]
[6]. A Choudry, N Bangash, A Malik, H Choudry, Adolescent ovarian tumors: A clinicopathological review of 15 cases;Ayub Med Coll Abbottabad 2008 20(4):18-21. [Google Scholar]
[7]. R Tandon, P Goel, PK Saha, N Takkan, RP Punia, A Rare ovarian tumor- Sertoli- Leydig cell tumour with heterologous element.Med Gen Med 2007 9(4):28-44. [Google Scholar]
[8]. Taxy Frozen Section and the Surgical PathologistArch Pathol Lab Med 2009 133:1135-38. [Google Scholar]
[9]. L Giuntoli, J Webb, Sertoli Leydig cell tumors of the ovary and pregnancy,CMEJournal of Gynecologic Oncology 2002 7:134-39. [Google Scholar]