Statins are competitive inhibitors of the enzyme HMG-CoA reductase, which catalyze an early, rate-limiting step in cholesterol biosynthesis [1]. Because of their safety, efficacy and tolerability these cholesterol lowering agents have become drug of choice for raised LDL-C in treating dyslipidemia [2]. A number of trials have demonstrated the efficacy of statins in reducing fatal and nonfatal CHD events, strokes, and total mortality [3].
Because of these factors, researchers have considered alternate day dosing for the primary prevention of CHD provided patients are not undertreated. That is. patients on alternate day therapy should be able to reach their goal LDL-C levels as per NCEP ATP III Guidelines as compared to the patients taking statins daily. A few studies have evaluated the efficacy and tolerability of alternate day statins and proved their ability to reduce health expense compared to daily day therapy [6,7].
Till date no such study has been done in the Indian population. Hence this study was undertaken to compare the efficacy and safety of daily versus alternate day 10 mg of Atorvastatin on reduction of lipid levels in naïve patients of dyslipidemia.
Methodology
This comparative randomized parallel group non-blinded study was done from January 2010 to April 2011 in 100 naïve diagnosed dyslipidemic patients needing treatment as per NCEP ATP III Guidelines [8]. The primary objective of the study was to compare the efficacy and safety of daily versus alternate day Atorvastatin 10mg on reduction of lipid levels in naïve patients of dyslipidemia. The study also evaluated the number of patients reaching LDL-C goals as per the NCEP ATP III Guidelines with both the treatments.
The study was approved by the institutional ethics committee and carried out according to the Indian Council of Medical Research (ICMR) Guidelines for Biomedical Research in Humans (2006); and in compliance with the International Conference on Harmonisation/Good Clinical Practice (ICH/GCP) Guidelines. Informed consent was obtained from the participants.
Adult Naïve patients of dyslipidemia in moderate or low risk group as diagnosed by NCEP ATP III Guidelines and patients willing to follow a low fat diet as advised by the investigator were included. These patients were classified into their risk category by using the online version of the 10-year risk calculator made available by The National Heart, Lung and Blood Institute based on the Framingham algorithm, (hin.nhlbi.nih.gov/atpiii/calculator.asp) / (hin.nhlbi.nih.gov/atpiii/riskcalc.htm) [9] [Table/Fig-1].
Adult Treatment Panel III LDL-C Goals in Different Risk Categories
| Risk Categories | Risk of developing CHD in the next 10 years | No of risk factors# | LDL goal (mg/dL) |
|---|
| Moderate Risk (M) | 10-year risk <10% | 2+ risk factors | < 130 |
| Low risk (L) | 10-year risk <10% | 0-1 risk factor | < 160 |
Risk factors# include cigarette smoking, hypertension (BP<140/90 mm Hg or on anti-hypertensive medication), Low HDL cholesterol (40 mg/dL), family history of premature CHD (CHD in male first-degree relative < 65 years of age), and age (men 45 years; women 55 years)
Patients with a history or clinical evidence of myocardial infarction, unstable or stable angina, coronary artery procedures, or myocardial ischemia; patients with history or clinical evidence of noncoronary forms of atherosclerotic disease; patients with uncontrolled diabetes or hypertension; impaired hepatic function were excluded from the study. Similarly patients with current use of lipid lowering drugs, Cytochrome P450 3A4 (CYP3A4) inhibitors or oral corticosteroid; undergone recent major surgery; or those who participated in any clinical trial in the past six months; pregnant/lactating mothers and women of childbearing potential, as well as patients with unstable medical or psychological condition were not included.
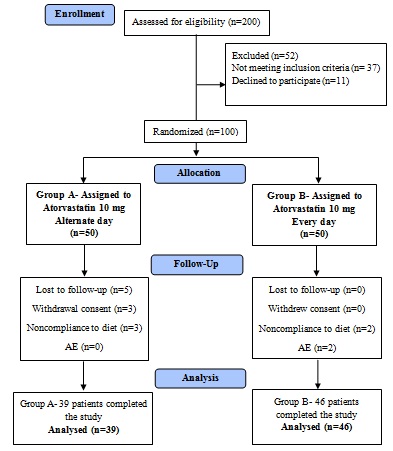
Of the 200 eligible patients assessed, 100 patients who fulfilled the study criteria were randomly allocated into two equal groups, using a computer generated randomization chart. Based on the calculations from Matalka MS study, [7] sample size calculations were done. The formula used for sample size calculation was as follows:
Formula used sample size per group = 1+2C(S/D) 2 C = constant (for alpha = 0.05 and power = 80%, C = 7.58); S = standard deviation (for LDL-C = 30, for HDL-C = 14, for TC = 44): D = difference to be considered significant (taken as 20% of mean i.e. for LDL-C = 22, for HDL-C = 10, for TC = 38) Sample size/group = 28.74 (for LDL-C), 30.71 (for HDL = C), 21.32 (for TC).
Though a sample size of 31 patients per group was ideal, it was decided to include 50 per group considering the possibility of loss of patients during a clinical trial.
Group A (n=50) received Atorvastatin tablets 10 mg every alternate day for three months and Group B (n=50) received Atorvastatin 10 mg daily for three months. Refer [Table/Fig-2] for the study flow details.
After obtaining general history, a clinical examination was done. Anthropometry measurements (height, weight, BMI) were done before and after the study. Baseline investigations included haemoglobin, blood sugar (fasting and postprandial) serum glutamic oxaloacetic transaminase/serum glutamic pyruvic transaminase (SGOT/SGPT) and Serum creatinine. Lipid profile TC, TG (Triglycerides), LDL, VLDL, HDL (High Density Lipoproteins) was done at the end of four weeks and 12 weeks to ensure compliance. The base line demographics and CHD risk factors are represented in [Table/Fig-3].
Baseline demographic and CHD risk factors of treatment groups)
| Lipid profile parameters | Atorvastatin 10 mg alternate day (Mean ± SD) n=39 | Atorvastatin 10 mg every day (Mean ± SD) n=46 | p-value |
|---|
| Age (years) | 48.13±9.671 | 48±8 | 0.9271 |
| Men | 18 | 22 | 0.8777 |
| Women | 21 | 24 | 0.8777 |
| Men ≥45 years | 16 ( 62.07% ) | 19 (41.30% ) | 0.9792 |
| Women ≥55 years | 11 (28.21% ) | 18 (39.13% ) | 0.4070 |
| Cigarette smoking | 15 (40.98%) | 17 (36.96%) | 0.8865 |
| Body weight (Kg) | 62.9744±8.7494 | 66.02±8.277 | 0.1032 |
| BMI (Body Mass Index) | 26.38±3.9366 | 27±3.57 | 0.4323 |
| Hypertension | 13 (33.34%) | 18 (39.13%) | 0 |
p-value for age was calculated using unpaired t test, p-value for men and women was calculated using Chi-square test with Yate’s correction, p-value for body weight, body mass index was calculated using unpaired t test, p-value for men> 45 years, women> 55 years, cigarette smokers, hypertension were calculated using Chi-square test with Yate’s correction
Every fifteen days patients were followed up for fresh drug supply, general evaluation and reporting of adverse events, if any. Efficacy was assessed by the percentage reduction in LDL and attainment of LDL goals as per NCEP ATP III Guidelines at the end of the study.
Of the 100 participants randomized to treatment, 85 completed the study and were analyzed by intention to treat analysis. For comparing quantitative data between the two groups post-treatment, Student’s unpaired t-test was applied. Comparison of non-parametric (qualitative) data between the study groups was done using Chi-square test with Yates correction or Fischer’s exact test two tailed p-value where applicable. P < 0.05 was considered statistically significant, and p < 0.001 was statistically highly significant.
Results
Out of 100 patients randomized, 85 completed the study. Out of the 15 patients who did not complete the study, five patients were lost to follow up; five patients discontinued due to noncompliance to diet; three withdrew consent, and two patients had treatment-emergent adverse events [Table/Fig-2].
Baseline characteristics and laboratory parameters
Both the groups had similar demographic profile at the start of the study [Table/Fig-3]. Similarly there was no statistically significant difference between the baseline laboratory parameters and lipid profile between the two groups at the start of the study. After 12 weeks of treatment, there were no important changes in haematology or biochemical laboratory values excepted for elevated liver enzymes in one patient on Atorvastatin 10 mg every day.
Lipid levels
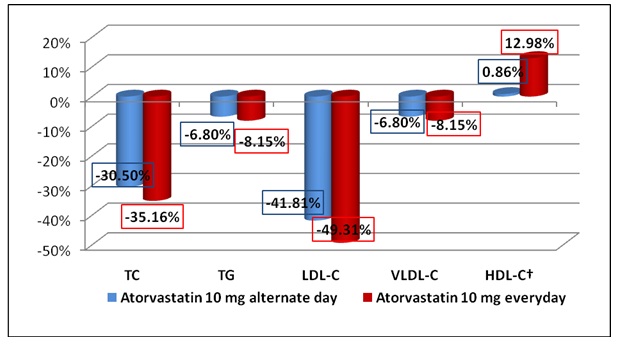
At the end of the treatment (week 12), there was a reduction in the levels of TC, LDL, VLDL and TG and increase in the levels of HDL compared to baseline [Table/Fig-4]. The [Table/Fig-5] depicts the mean reduction in lipid parameters in both the groups at week 12. It was seen that Atorvastatin 10 mg daily produced a significantly greater reduction in TC, LDL, VLDL as compared to Atorvastatin 10 mg alternate day .The increase in the HDL level was also significantly greater with a daily dose as compared to alternate day Atorvastatin. However the differences in TG were not statistically significant. The mean percentage change in lipid parameters in both the groups at week 12 is presented in [Table/Fig-6].
Baseline lipid parameters (mean ± SD) of the groups at 12 weeks
| Lipid profile parameters | Atorvastatin 10 mg alternate day (Mean ± SD) n=39 | Atorvastatin 10 mg every day (Mean ± SD) n=46 | p- value |
|---|
| TC | 246.15±37.89 | 258.48±30.86 | 0.1020 |
| TG | 135.25±62.51 | 141.15±29.65 | 0.5706 |
| LDL | 176.05±35.12 | 190.19±27.48 | 0.0405 |
| VLDL | 27.05±05.3 | 28.23±6.17 | 0.3517 |
| HDL† | 43.05±8.72 | 40.06±7.6 | 0.0949 |
p-value was calculated using unpaired t-test
Mean reduction in lipid parameters (Mean ± SD) of the groups at 12 weeks
| Lipid profile parameters | Atorvastatin 10 mg alternate day (Mean ± SD) n=39 | Atorvastatin 10 mg every day (Mean ± SD) n=46 | p- value |
|---|
| TC | 75.07±12.53 | 90.89±10.86 | <0.0001** |
|---|
| TG | 9.2±13.71 | 11.5±19.65 | 0.5404 |
| LDL | 73.6±14.71 | 93.79±17.48 | <0.0001** |
| VLDL | 1.84±0.63 | 2.3±0.17 | <0.0001** |
| HDL† | 0.37±0.14 | 5.2±0.6 | <0.0001** |
p-value was calculated using unpaired t-test
Mean percentage change in lipid parameters from baseline to week 12 (will become figure) († % increase for HDL)
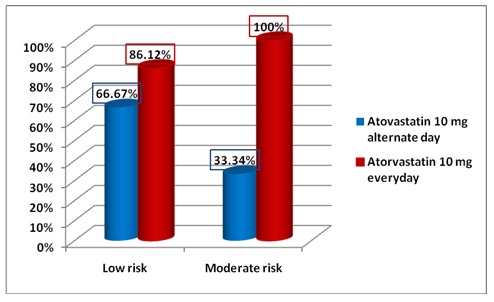
[Table/Fig-7] highlights the proportion of patients reaching LDL-C target at the end of the study (week 12). It is evident that in Low risk category 86.12% patients (n=31) on Atorvastatin 10 mg daily reached their LDL-C goal as compared to 66.67% (n=18) of patients on alternate day therapy. In the moderate risk category 100% of the patients (n=10) on Atorvastatin 10 mg daily achieved their LDL-C goal as compared to 33.33% patients (n=4) on alternate day therapy. The same is depicted in [Table/Fig-8].
Proportion of patients reaching LDL-C target by the end of the study
| Risk category | Target LDL level | Atorvastatin 10 mg alternate day n=39 | Atorvastatin 10 mg every day n=46 | p-value |
|---|
| Total number of patients | Number (%) of patients reaching target | Total number of patients | Number (%) of patients reaching target |
|---|
| Low Risk (L) | 130 mg/dl | 27 | 18 (66.67%) | 36 | 31 (86.12%) | 0.0774 |
| Moderate Risk (M) | 160 mg/dl | 12 | 4 (33.33%) | 10 | 10 (100%) | 0.2406 |
p-value was calculated using Fischer’s test
Percentage of patients reaching target LDL levels
Adverse events
There were no serious adverse events during treatment in both the groups. There were a total of 10 adverse events in the group receiving Atorvastatin 10 mg daily vis-a-vis four adverse events in the group receiving alternate day therapy [Table/Fig-9]. Headache, asthenia, dyspepsia, dizziness, paraesthesia, depression, myalgia and elevated liver enzymes were the reported adverse events. These were mild in intensity. Only the patients reporting myalgia (n=1) and elevated liver enzymes (n=1) were discontinued from the study.
Incidence of adverse events
| Incidence of AE | Atorvastatin 10 mg alternate day | Atorvastatin 10 mg every day |
|---|
| Total | 4 (39.34%) | 10 (44.83%) |
| Headache | 1 | 1 |
| Asthenia | | 1 |
| Digestive system |
| Constipation | | |
| Diarrhoea | | |
| Dyspepsia | 1 | |
| Nausea | | |
| Flatulence | | |
| Nervous system |
| Dizziness | 1 | 2 |
| Parasthesia | 1 | 3 |
| Depression | | 1 |
| Musculoskeletal system |
| Myalgia | | 1 |
| Elevated liver enzymes | | 1 |
Discussion
This study compared the efficacy and safety of Atorvastatin 10mg alternate day versus daily treatment for primary prevention of CHD in naïve dyslipidemic patients. Our results show that daily Atorvastatin provides a statistically significant advantage in LDL-C reduction as well as in the percentage of patients able to reach the NCEP ATP III LDL-C targets compared with alternate day therapy.
Despite being less efficacious than the daily regimen, this alternate day dosing regimen still provided an LDL-C reduction of 41.81%, which would be comparable to LDL-C reductions achievable with less potent statins such as fluvastatin, lovastatin and pravastatin. The observation that reduction of 66.67% in the low risk category and 33.34% in the high risk category in LDL-C target goals suggests that Atorvastatin 10 mg alternate day dosing has a potential role in clinical practice.
Contrary to our results some researchers have documented that alternate day Atorvastatin treatment is on par with daily therapy.
Matalka et al., studied 35 hypercholesterolemia patients in a double blind, placebo controlled design for six weeks. In the 26 patients who completed the study they found that alternate day Atorvastatin produced a reduction in LDL-C that was comparable to daily administration. Besides this, the alternate day therapy was less expensive. They further found that after six weeks, if the dose of patients who were not at LDL-C goal was doubled, the LDL-C reductions were 27% in the alternate day and 38% in the daily groups respectively. In this study, the patients in both the study groups did not experience myalgia, elevation of creatinine kinase levels or hepatotoxicity. It was also observed that patients on alternate day therapy paid 34% less than daily therapy patients annually [7].
In a similar study to ours, Piamsomboom et al., [10] evaluated efficacy and safety of alternate day 10 mg Atorvastatin for eight weeks in hypercholesterolemia patients. It was seen that there was a significant reduction in cholesterol levels with the alternate day dosing. The noted adverse drug reactions were abnormal liver function, increase in creatine kinase levels and somnolence, all noted in one patient each [10].
Jafari et al., conducted a prospective, non blinded, controlled clinical trial in 54 patients randomized to receive 10 mg Atorvastatin daily; 10 mg Atorvastatin alternate day; and 20 mg Atorvastatin alternate day. Although all the three regimens significantly reduced TC and LDL- C compared to baseline, the decrease was not statistically significant. All regimens were well tolerated and none of the patients had a significant elevation of liver enzymes or creatine kinase. They concluded that alternate-day Atorvastatin is an efficacious and safe alternative to daily dosing [6].
Juszezyk et al., did a retrospective analysis of 25 patients who were either on Atorvastatin (10 mg daily; 10 mg alternate day; 40 mg daily) or Rosuvastatin (5 mg alternate day; 10 mg alternate day) for at least one month. They showed that 12/15 patients who were previously intolerant to Atorvastatin daily therapy due to myalgias tolerated alternate day therapy for more than one month [11].
Ferrer-Garcia et al., [12] in their prospective trial on Atorvastatin every other day in 44 type 2 diabetic patients showed an 8.4% difference in TC and a 15.8% difference in LDL-C. Out of the 33 patients who completed the study, none of the patients showed elevations in liver enzymes or creatine kinase in the alternate-day group versus one patient in the daily treatment group who experienced elevated liver enzyme levels and was withdrawn [12].
Aghosadeghi K et al., randomized 60 patients on Atorvastatin to three groups; Group 1- 10 mg daily, Group 2- 20 mg daily and Group 3- 20 mg every other day. Their results showed no significant difference after six weeks treatment in all the three groups. There was a similar reduction in total cholesterol and LDL-C as compared to baseline in all the three groups. There was no incidence of elevated liver enzymes or creatine kinase levels [13].
Similarly, Ghattas and Pimenta in their study in 100 hypercholesterolemia patients concluded that a cost reduction between 30% and 50% was observed if the weekly dosage of Atorvastatin was reduced [14]. Keles et al., in their prospective, randomized study compared Atorvastatin 20 mg daily versus 20 mg alternate day in 61 dyslipidemic patients with moderate to high risk of cardiovascular disease. They concluded that both Atorvastatin 20 mg daily and alternate day had similar efficacy after 1 and 3 months of therapy. Also, there was no significant difference in reduction of high-sensitivity C-reactive protein (hsCRP) between the two groups (p>0.05) [15].
Though statins play a central role in cardiovascular risk reduction, only half the patients prescribed a statin adhere to this therapy [16]. Non adherence is the main reason why patients do not achieve LDL goals, and in effect have worsened clinical outcomes [17] and higher healthcare costs than their adherent counterparts [18]. For some patients, side effects lead to discontinuation, while others misunderstand the importance of statin therapy because of the asymptomatic nature of hyperlipidemia [19].
Previous studies compared the efficacy and safety of daily therapy versus alternate day therapy with Atorvastatin have suggested both the dosing patterns to be equally safe, efficacious and less expensive [6,7,10,11,12,13,15]. As observed in our study, the compliance of patients in the alternate day group was much poor as they forgot to take the medication. Also some patients foster the notion that since the medication is to be taken alternate day it is not a vital medicine and one or two missed doses will not be of any harm. It was also noted that elderly dyslipidemic patients tend to forget their medication. Against this, the compliance with a daily dose will be much better.
Though a starting dose of 10 mg is considered as the standard of care for Atorvastatin; in India 5 mg dose of Atorvastatin is widely marketed. Hence, for patients experiencing side effects prescribing a 5 mg dose may be a better alternative than giving alternate day therapy in order to ensure compliance. However, in order to provide cost saving, compromising in achieving goal LDL-C levels is definitely not advisable.
Conclusion
Atorvastatin in a dose of 10 mg daily was found to be safe and efficacious in adult patients with dyslipidemia compared to an alternate day therapy. Looking at the number as well as percentage of patients reaching LDL-C goals as per the NCEP ATP III Guidelines, it can be said that daily therapy with Atorvastatin was better. In our set of patient population, adherence to treatment can be a challenge and daily therapy with Atorvastatin would be a better option. Alternate day Atorvastatin may be reserved for patients intolerant to statins and when doses lower than 10 mg are not available.
Risk factors# include cigarette smoking, hypertension (BP<140/90 mm Hg or on anti-hypertensive medication), Low HDL cholesterol (40 mg/dL), family history of premature CHD (CHD in male first-degree relative < 65 years of age), and age (men 45 years; women 55 years)
p-value for age was calculated using unpaired t test, p-value for men and women was calculated using Chi-square test with Yate’s correction, p-value for body weight, body mass index was calculated using unpaired t test, p-value for men> 45 years, women> 55 years, cigarette smokers, hypertension were calculated using Chi-square test with Yate’s correction