From 2006 to 2010, an annual average of 436.8 men and 318.2 women developed malignant tumours of the oral cavity and/or pharynx. Their median age was 62.9 years. This corresponded to an incidence of 0.00953% per year in the Swiss population, which was numbered 7,870,134 people in 2010. 28.2% of the Swiss population are active smokers and 21.5% have a smoker history (Federal Statistics Office, www.bfs.admin.ch). Most of the tumours of OSCC are found in the mouth (88%), but it can also be found on the lips (8%), and in the oropharynx (4%) [1]. During the past 50 years, great progress has been made in surgical treatment and radio - and chemotherapy, but the rate of mortality has not improved sufficiently [2]. For this reason, potentially malignant disorders which are at a risk of developing into OSCCs such as oral leukoplakia, erythroplakia, erythroleukoplakia or premalignant conditions such as oral lichen planus and oral submucous fibrosis, need to be detected and diagnosed as early as possible and followed up over a long term, to prevent malignant transformation. Different methods are available for collecting cytological samples for analysis: exfoliative cytology, brush biopsy (BB), and scalpel biopsy (incisional or excisional) depending upon the site, size and severity of lesion [2].
Brush biopsy is a minimal invasive method by which cells of all epithelial layers are obtained. It contains even small epithelial tissue fragments which can be embedded in paraffin and examined as histological specimens (called “cell block method”). For this special procedure, the Paraffin-gelatine processing validation has been developed and it has been used in the Institute of Pathology, Reutlingen, Germany [3].
The Oral CDx® BB method is a computer-assisted sample analysis of the cytologcial smear that detects abnormal cells in all cell layers of the epithelium of the oral mucosa. The results are presented to a cytopathologist as a cell gallery [4]. The basal layer gets damaged less than it gets through an SB, so that healing takes place as a regeneration ad integrum. The procedure can be used without any special training, thus making it popular among professionals. The biological potential of the oral epithelial cells which are obtained can be evaluated also by the following additional methods: DNA cytometry, immunohistochemistry, monolayer cytology, and molecular biological analysis. Uptil now, further investigations were needed to check as to how far the additional methods helped in increasing the sensitivity and specificity of oral BB [4].
Oral CDx® BB can aid in confirming the nature of potentially malignant disorders and for revealing those that are precancerous and cancerous when they are not clinically suspected of being so [2]. Because of the frequent occurrence of oral lesions, it would be disproportionate to undergo invasive biopsies. The aim of this study was to examine as to whether the brush biopsy and subsequent computer-assisted analysis were useful as a screening method for dentists in private practice.
Materials and Methods
Within 60 months (from 2003 to 2008), 263 brush biopsies were performed in 200 patients with potentially malignant disorders at the stomatology service of the Department of Oral Surgery, at the University of Zurich, Switzerland. Malignant suspicious lesions were excluded from the study and they directly underwent SB. Also, ulcerative lesions no longer contain a complete epithelium and thick hyperkeratotic conditions, whose epithelial layers are impossible to reach, were not indicated.
Patient Profiles
Socio-demographic data were collected (gender, age, smoking behaviour) for all the 200 patients. The patients were examined according to a standardized protocol: extra-oral inspection with a focus on symmetry and status of the lymph nodes. The intraoral examination recorded lesions that were clinically suspicious for potentially malignant disorders [Table/Fig-1]. The clinical diagnoses have been shown in [Table/Fig-2]. Contraindications for the BB were lesions that were highly suspicious for malignancy, those which had no intact epithelium or lesions with an intact epithelium, such as submucosal masses, pigmented lesions, fibromas, and mucoceles. We collected information concerning their morphologies, colours, localizations, extensions, and clinical diagnoses. Clinical photographs were taken from all mucosal lesions at the initial- and the follow up examinations.
Clinically suspicious for potentially malignant disorder (lichen ruber mucosae with multiple clinical parameters)
OralCDx® results according to the clinical diagnosis
| Clinical diagnosis | Negative | Atypical | Positive | Inadequate | BBs |
|---|
| Leukoplakia | 115 | 21 | 5 | 5 | 146 |
| Hyperkeratosis | 31 | 1 | - | 2 | 34 |
| Ulcerative changes | 8 | 4 | 1 | 1 | 14 |
| Erythroleukoplakia | 9 | 1 | 1 | - | 11 |
| Mech. / chem. Irritation | 10 | - | - | 1 | 11 |
| Lichen ruber mucosae [5] | 8 | 1 | - | - | 9 |
| Lichen reticularis | 3 | - | - | - | 3 |
| Lichen erosivus | 5 | 1 | - | - | 6 |
| Atrophic Lichen | 1 | 2 | - | 1 | 4 |
| Erythroplakia | 6 | 1 | - | - | 7 |
| Smoker’s keratosis | 5 | 1 | - | - | 6 |
| Erosive changes | 2 | 2 | - | - | 4 |
| Candidiasis | 2 | 2 | - | - | 4 |
| Induration | 1 | - | - | 2 | 3 |
| Irritation fibroma | - | 1 | - | 1 | 2 |
| Lingua geographica | 2 | - | - | - | 2 |
| Scar tissue | 2 | - | - | - | 2 |
| Aphtoide lesion | - | 1 | - | - | 1 |
| Papillary hyperplasia | - | 1 | - | - | 1 |
| Glossitis rhombica mediana | 1 | - | - | - | 1 |
| Lingua plicata | 1 | - | - | - | 1 |
| Total | 204 | 39 | 7 | 13 | 263 |
Clinician Profiles
For the examination of the patients, the BBs and the SBs, 32 clinicians with specialization for oral surgery were involved. All 32 clinicians worked at the stomatology service of the Department of Oral Surgery at the University of Zurich, Switzerland and they were trained for diagnosing mucosal lesions. The clinicians where grouped into three levels of experience: low-experienced and experienced clinicians, and experts. SBs were performed by the same clinician who performed the BBs.
OralCDx® Brush Biopsies
The potentially malignant disorders were biopsied by using the OralCDx® testkit [Table/Fig-3] according to the manufacturer’s guidelines. Cells from all cell layers, including the basal membrane, were collected with a moistened brush by doing 5-10 rotations. The cell samples were fixed on the slides and they were air dried. Also, the brush was stored in a tube with fixation fluid. The material was sent to the Pathological Institute of the District Hospital, Reutlingen (Prof. Dr. A. Burkhardt), where the cells were stained with Papanicolaou’s stain and analyzed with a neural network-based image processing system which was specifically designed to detect oral precancerous and cancerous cells. The results were classified into four groups (Sciubba 2001):

Negative: No epithelial abnormality.
Atypical: Abnormal epithelial changes of uncertain diagnostic significance.
Positive: Definitive cellular evidence of epithelial dysplasia or carcinoma.
Inadequate: Incomplete transepithelial biopsy specimen.
Abnormal cells (n=192) were re-analyzed by an experienced cytopathologist. If it was deemed necessary and the cells were also examined under the microscope. In addition, a cell block was obtained from the tissue material which was on the brush, cut at 6μ and stained with the haematoxylin and eosin (HE) and periodic acid Schiff (PAS) methods. These slides were examined manually by the pathologist and they were taken into consideration for the final diagnosis, which was given to the clinician.
Scalpel Biopsies
The period of time when the SBs were performed, depended on the clinical appearances and the results of the BBs. Patients underwent SBs either simultanously with the BBs or in the follow-up visits. In the case of atypical or positive results, SBs were performed when the diagnoses were obtained. For negative results, the SBs depended on future accurancies. The longest time interval between a BB and the corresponding SB were 3 months. They were processed without having any knowledge on the results of the brush biopsies at an independent Institute of Pathology.
Statistical Analysis
Only the cases which underwent both procedures, BBs and SBs, were taken up for statistical analysis. The analyses were conducted by using SPSS Statistics, version 18. To calculate the sensitivity and specificity, all inadequate BB samples (n = 13) were excluded. Atypical and positive BB results were summarized as positive results [6]. The histological diagnoses of the SBs were grouped as “negative” in cases of no dysplasia and as “positive” if cells with dysplasia or malignant tumour cells were present. For measurements of sensitivity and specificity, 95% confidence intervals (CI) were included. Also, the negative predictive value (NPV) and positive predictive value (PPV) were calculated.
Results
Patient Profiles
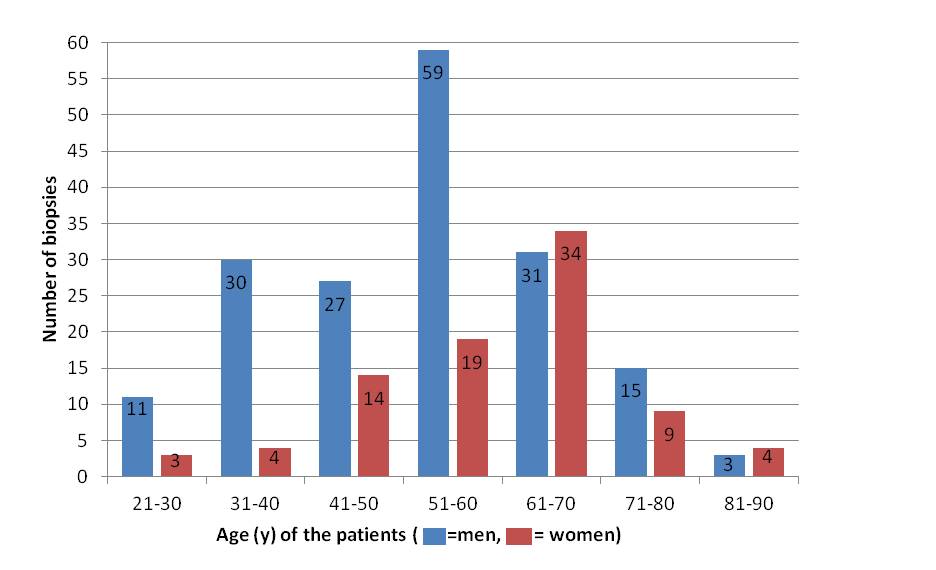
A total of n=263 BBs were collected by 32 clinicians from 200 patients (87 biopsies from women, 33.1%; 176 from men, 66.9%) (Table/Fig-4]. Most of the patients were between 51 and 60 years of age (78/200, 39%); this age group consisted mostly of men (n=59; 33.5% of all BBs in men). The maximum number of BBs for women was found in the 61-70 age group (n= 34; 39% of all BBs in women).
Age and gender distribution of the brush biopsies (n = 263)
Biopsy Sites
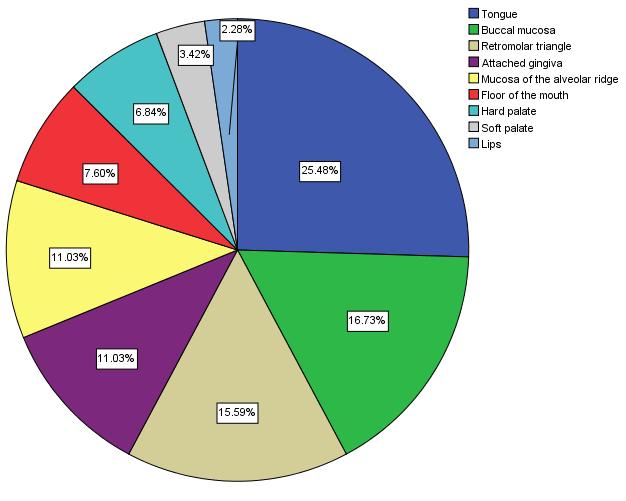
The most common site for BBs was the tongue (25%), followed by the buccal mucosa (17%), retromolar triangle (16%), attached gingiva (11%), mucosa of the alveolar ridge (11%), floor of the mouth (8%), and hard palate (7%). Fewer biopsies were taken from the soft palate (3%) and the lips (2%) [Table/Fig-5].
Distribution of the oral locations of the brush biopsies (n = 263)
Classification of Brush Biopsies
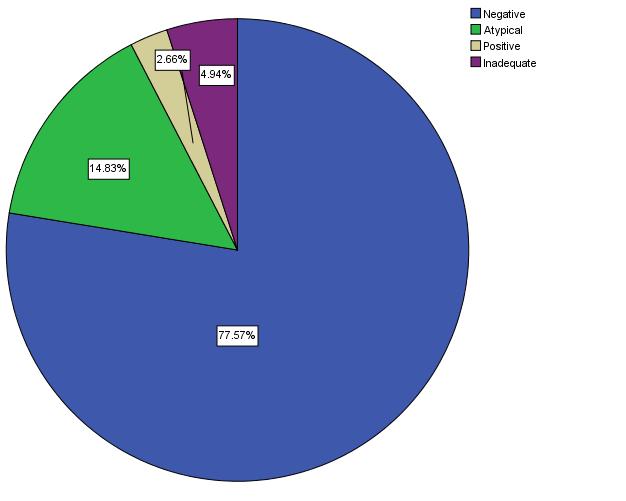
The 263 BBs were analyzed and classified into the four groups, as has been described in the Methods section: 204 (78%) were negative, 39 (15%) were atypical, 7 (3%) were positive, and 13 (5%) were inadequate [Table/Fig-6]. No significant difference in biopsy results was found between men and women. However, the percentage of “atypical” results increased continuously from the youngest age group to the oldest one. In contrast, the percentage of “negative” results decreased with age [Table/Fig-7,8]. “Positive” results were found only in the age groups of 41-50 (5.1%) years, 51-60 (5.4%) years, and 61-70 (1.6%) years, with a peak in the fifth decade.
Classification of the brush biopsies (n = 263)
Increase in number of “atypical” results (n = 39) with age
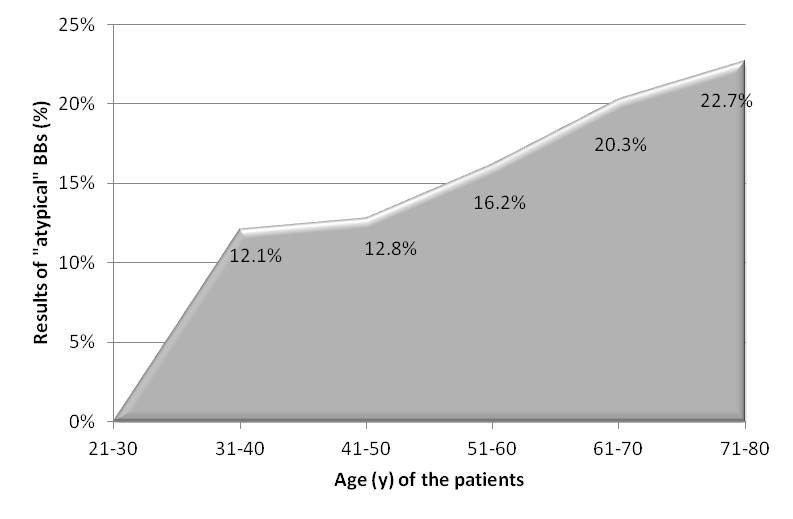
Decrease in number of “negative” results (n = 204) with age
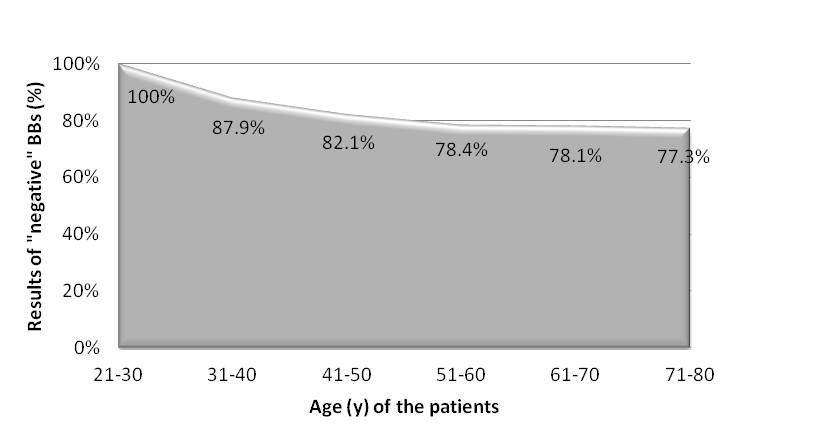
Neither the buccal mucosa nor the retromolar triangle showed “positive” findings, although these were the most biopsied regions, besides the tongue [Table/Fig-9]. Most of the epithelial dysplasias were found in the floor of the mouth (16%), soft palate (11.1%), and hard palate (6.3%). The hard palate showed a significantly higher occurrence of atypical and positive cell changes than all other oral sites (p = 0.016).
OralCDx® results for the various biopsied sites
| Localization | Negative | Atypical | Positive | Inadequate | BBs |
|---|
| Tongue | 50 | 13 | 1 | 3 | 67 |
| Buccal mucosa | 39 | 4 | 0 | 1 | 44 |
| Retromolar triangle | 35 | 3 | 0 | 3 | 41 |
| Gingiva | 23 | 4 | 1 | 1 | 29 |
| Mucosa of the alveolar ridge | 25 | 4 | 0 | 0 | 29 |
| Floor of the mouth | 12 | 3 | 3 | 2 | 20 |
| Hard palate | 9 | 6 | 1 | 2 | 18 |
| Soft palate | 6 | 2 | 1 | 0 | 9 |
| Lips | 5 | 0 | 0 | 1 | 6 |
| Total | 204 | 39 | 7 | 13 | 263 |
Before biopsies, 19 different clinical diagnoses were made by the clinicians. The most common diagnosis was leukoplakia (55%) [Table/Fig-2]. However, even though leukoplakias were biopsied most often, the relative frequency of occurrence of epithelial dysplasia (“Positive”) was higher in erythroleukoplakia (1/11, 9.1%) and in ulcerative changes (1/14, 7.1%) than in leukoplakia (5/146, 3.4%) [Table/Fig-9].
Relevance of Smoking Behaviour
Almost half (49.5%) of the BBs were taken from smokers. We found no significant correlation between smoking behaviour and the OralCDx® results. The proportion of smokers (4/142, 2.8%) was similar to that of nonsmokers (3/121, 2.5%) for positive results. For atypical results, the percentage of non-smokers was even a little higher (21/121, 17.3%).
Clinician-specific Bias
The results of the BBs and SBs did not show significant differences in comparison to the three experience levels of the clinicians.
Comparison with Scalpel Biopsy
All 7 “positive” and 29 “atypical” BBs were compared with scalpel biopsies [Table/Fig-10]. Ten patients with atypical cell changes were not biopsied, because either there was no longer any apparent mucosal change or the patients had refused scalpel biopsies. The clinicians decided to perform scalpel biopsies in 17 of the negative BBs and in all 3 inadequate BBs. The histological results showed that all positive BBs contained epithelial dysplasia, and that 3 were even OSCCs. Of the atypical BBs, 17.2% were modified to mild dysplasia; 6.9% were modified to moderate dysplasia; 3.4% were modified to severe dysplasia; and 6.9% were diagnosed as OSCC. Two of the negative BBs were false-negatives—one was a mild dysplasia and the other was a moderate one.
Comparison of OralCDx® results with scalpel biopsies
| Scalpel biopsy diagnosis |
|---|
| OralCDx® diagnosis | BBs | No dysplasia | Mild dysplasia | Moderate dysplasia | Severe dysplasia | Oral squamous cell carcinoma | Scalpel-biopsies |
|---|
| Negative | 204 | 15 | 1 | 1 | - | - | 17 |
| Atypical | 39 | 19 | 5 | 2 | 1 | 2 | 29 |
| Positive | 7 | - | 1 | - | 3 | 3 | 7 |
| Inadequate | 13 | 2 | - | - | - | 1 | 3 |
| Sum | | 36 | 7 | 3 | 4 | 6 | 56 |
| Percentage [%] | | 64.3% | 12.5% | 5.4% | 7.1% | 10.7% | 100% |
The comparison of BBs and scalpel biopsies achieved a sensitivity of 90% (CI: 69%-97%) and a specificity of 44.1% (CI:29%-61%). The PPV was 47.2%; and the NPV was 88.2%.
Discussion
Currently, the early detection of precancerous or cancerous lesions seems to be the only way for reducing the high mortality rate of head and neck cancers, especially of cancers of the oral cavity. OralCDx® brush biopsy is a technique that can be used by every dental clinician to determine as to whether oral lesions contain atypical or dysplastic cells; consequently, the scalpel biopsy becomes a second diagnostic procedure that is only needed in a further step [7,8].
Oral cancer is more frequent in men than women. Depending on the location of the cancer within the oral cavity, men are two to six times more likely to be affected than females, which largely owes to their higher intake of alcohol and tobacco [9]. Also, in our study, more men than women had potentially malignant disorders which needing BBs, which was expected. (Two thirds of the BBs in our study were taken from men and one third was taken from women.) However, we did not find a significant association between biopsy results and gender.
The predilection sites of OSCC are the tongue and the floor of the mouth [10]. These sites accounted for 33% of the BBs in this study. We found cell abnormalities in 25% of the biopsies which were taken from the tongue and in 40% of the biopsies which were taken from the floor of the mouth. In addition, the occurrence of atypical and positive BB results was significant for the hard palate.
The inadequate results (5%) seemed to be relatively high. They were biopsied again, either through BBs or SBs. But all in all, it showed that the effectiveness of the BBs depended on the thickness of the epithelial layers.
In the literature, sensitivity ranged from 92.3% [11] to 71.4% [12]; specificity ranged from 100% [13] to 32% [12]; the PPV ranged from 95.7% [14] to 7.9% [15]; and the NPV ranged from 60% [12] to 97% [11]. The PPV for the atypical findings was 42.9% and that for the positive findings was 100%, as was seen in a former study [16]. It is difficult to evaluate our data in relation to the literature, because there are great differences in how the parameters are calculated. Hohlweg-Majert et al., [6], for example, summarized the atypical and positive cell abnormalities to calculate the sensitivity and specificity; other authors [12,17] calculated them separately. The sensitivity of our findings was remarkably high if one considered that we included also the atypical results of the BBs. Atypical results may be histologically positive for dysplasia or they may only represent reactive processes caused by regeneration (ulcer) or infection (candidosis), etc. [18]. None of the 7 positive BBs that required scalpel biopsies were false-positive, meaning that the sensitivity would be 100%. This was in accordance with the results of the former study done by Kosicki et al., [16].
OralCDx® cytologic test has been clearly shown to be highly sensitive and specific for detecting dysplastic epithelial changes in clinically high-risk lesions, but when it was used in low-risk lesions, the accuracy was reduced and the rate of false-positive findings had increased [19].
The “gold standard” of diagnosis was to compare BB results with those of scalpel biopsies. The BB is regarded as an alternative method for scalpel biopsy. But is this the right approach? Many clinical trials and reports have shown positive indications for the prospective roles of these methods as additional aids in the early detection of oral cancer. A vast majority of these studies concentrated on diagnostic test methods rather than the use of these tests for screening [20]. Generally, most of the studies like ours, which used the BB as a screening tool [2,12,21], had similar limitations. Statistical analyses could only be performed for patients who underwent BBs and SBs. But there is a contradiction between the study accuracy and the medical necessity of an invasive SB for many patients, especially in a study that goes over several years. Furthermore, many clinicians were involved in our study. Our results showed that there was no significant effect due to the level of training and experience of the clinicians. Another weakness was the different periods of time between the BBs and SBs, which is a necessity for proper research results, but these cannot be performed over years and in the daily routine of a stomatology service. The advantage of these weaknesses is that they present realistic conditions and that they show a high number of cases. The next question is as to how far a study of the stomatology service of the Department of Oral Surgery at the University of Zurich was comparable to a private practice. But the study of Bhoopathi et al., [22] showed that oral surgeons’ effectiveness in diagnosing oral dysplastic lesions was only slightly better than that of the OralCDx®BB. Uptil now, the survival of OSCC patients has still not improved. The 5-year survival rate was 80% in cases which were detected at the initial stage, it was 40% in cases which had regional involvement, and it was less than 20% in cases with distant metastasis [23]. Detection of a potentially malignant disorder is the key to preventing mucosal alterations and thus, decreasing the risk of malignant transformation [24].
Conclusion
For the sensitivity and specificity of BBs, there is still space for improvement, but they are already high. Additional methods like DNA-image cytometry may enhance the results and this has to be investigated in further studies. But the present study showed that potentially malignant disorders can be detected with this noninvasive technique. So, we conclude that it would be a helpful method, especially for non-specialists and in the daily routine of a dental practice.
Authors’ Contributions
SC, JMB drafted the manuscript and did the statistics. AB, as an cytopathologist, participated in the design of the study and checked the histological part. MCL initiated the study, supervised the thesis of MAT and participated in the design and coordination of the paper. MAT carried out the retrospective study.