The demand for tooth whitening has increased dramatically over the past few years. Since then, several agents were used directly or indirectly to act upon teeth. Considering efficacy, advantages and disadvantages of different materials, most commonly used materials are hydrogen peroxide, carbamide peroxide and sodium perborate [1, 2].
Mechanism of dissolution of glass ionomers was explained as follows: In acidic medium, H+ ions diffused into cement and they were replaced with metal cations that crosslinked the polycarboxylic acid molecules in the cement matrix. According to the concentration gradient, the free metal cations would diffuse through the cement outword and be released from the surface. As the metal cations in the cement matrix decreased, they were extracted from the glass particles into the matrix. The extraction of metal cations caused an increase in non-bridging oxygen in the network of glass near its surface [3–6].
Bleaching gels and glass ionomers have many possibilities for interacting in the oral cavity during bleaching. It is important to understand such an interaction [7].
Although bleaching is safe for soft tissues from a procedural standpoint, but it may not be safe for dental materials. When hydrogen peroxide interacts with dental materials, it decomposes to form hydroxyl radical intermediates and finally, to form water and oxygen [8,9]. Also, carbamide peroxide will dissociate to H2O2, CO2, urea and NH3, and then, H2O2 will decompose again to water and oxygen. These chemical ingredients may affect the dissolution of glass ionomer restorations [10].
Materials and Methods
Time and Place
This research was performed in cooperation with the faculty of dentistry, Ain Shams University, in 2010 and it was funded by the authors.
Sample Size and Division
Ninty samples were divided into three groups (30 samples for each type of glass ionomer).
Each group was further divided into three subgroups; the first subgroup was control samples, the second subgroup was bleached by the first bleaching material and the third subgroup was bleached by the second bleaching material.
Materials Used
Two vital bleaching commercial products and three types of glass ionomer restorative materials were selected for this study. Bleaching materials used were Opalescence Xtra Boost (38% hydrogen peroxide with pH of 7) and Opalescence Quick (35% carbamide peroxide with PH of 6), both of which were manufactured by (Ultradent Inc., South Jordan, Utah, USA).
Glass ionomer materials used were Ketac Fil (conventional glass ionomer), Photac Fil (resin modified glass ionomer) and F2000 (poly acid modified composite resin), which were manufactured by 3M (Espe. StPaul, USA). Shade of all glass ionomer materials used was A2.
Procedure
Thirty disc shaped specimens (5 mm in diameter and 2 mm in thickness) were prepared for each brand of glass ionomer. A Teflon mold was used for sample preparation. The mold was sandwiched between two glass plates to allow setting of glass ionomers under pressure.
Capsules of Ketac Fil were activated and then triturated according to manufacturer’s instructions for 15 seconds and they were injected into the holes of the mold in one increment. The mold was filled to slight excess. The specimen’s top surface was covered by a Mylar strip and a glass slide was secured to flatten the surface. It was pressed by using a standard load of 500 mg over the mold and it was then left for setting.
Capsules of both Photac Fil and F2000 were triturated according to manufacturer’s instructions for 15 seconds and they were injected into holes, covered with glass slides and light cured for 40 seconds per each side by using a light source (Pencure, J Morita MFG corp., Japan).
Prior to testing, the specimens were incubated in a 95% relative humidity environment at 37°C for 24 hours. After 24 hours, the ninety samples were removed from water, thoroughly dried with absorbent paper, placed in a dehumidifier for 24 hrs and weighed on an HM-200 precision scale (A and D Company Limited, MA, USA). Their weights were recorded as weights before the test T0.
Control samples were returned back to their tubes after replacing water with fresh distilled water, 5 ml.
Bleaching with Opalescence Xtra was done in three applications of 15 minutes each, without light activation, by following the manufacturer’s instructions. Each sample was covered by 2 ml of the bleaching material and after each application, the specimens were washed with distilled water and dried with oil-free compressed air.
Bleaching samples with Opalescence Quick was done by applying 2 ml of the material over each sample for 60 minutes according to manufacturer’s instructions. All bleached samples were washed thoroughly with distilled water and were returned back to their tubes after replacing water with 5 ml of new distilled water.
Follow-Up
After one week, samples were removed, thoroughly dried with absorbent paper, placed in a dehumidifier for 24 hrs, weighted and returned back; weights were recorded for each specimen. Again the same procedure was repeated after one month and three months. Every time samples were rinsed with distilled water and water in the tubes was exchanged with new 5 ml of distilled water. The weights of samples were expressed in grams.
The weight loss of each sample, which was expressed as percentage of the original mass, was considered to correspond to the solubility and disintegration of the cement, which was calculated according to this equation: D% = % (W0 – Wt)
Where:
D%: Disintegration percentage.
W0: Weight of samples before test.
Wt: Weight of samples after test.
Statistical Analysis
Data for dissolution were analyzed by using one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test at a significance level of α = 0.05. The analysis of variance was carried out by considering the factors: material, time, and interaction.
Results
The results showed that time had a highly significant effect on dissolution of control samples of all materials (p < 0.01). It also indicated that weights of all samples decreased by T1 (after one week) and that they then became nearly constant through all other periods of the test (one month and three months) [Table/Fig-1]. The control group showed more dissolution percentage in Ketac Fil than in Photac Fil and F2000 in the first period of the test, T1. No difference was detected between the three materials during the remaining period of the test.
Mean and standard deviation (n=30) of weight (g) of all control samples through each period of the test.
| Mean ±SD | Time | Material |
|---|
| 0.15 ±0.007 | T0 | Ketac Fil |
| 0.092 ±0.004 | T1 | G1 |
| 0.098 ±0.004 | T2 | |
| 0.104 ±0.005 | T3 | |
| 0.14 ±0 | T0 | Photac Fil |
| 0.094 ±0.005 | T1 | G2 |
| 0.094 ±0.008 | T2 | |
| 0.096 ±0.008 | T3 | |
| 0.122 ±0.004 | T0 | F2000 |
| 0.082 ±0.008 | T1 | G3 |
| 0.08 ±0.007 | T2 | |
| 0.078 ±0.008 | T3 | |
T0=Weight of the samples before test (after 24 hours) - T1= Weight of the samples after one week - T2= Weight of the samples after one month - T3= Weight of the samples after 3 months
When dissolution percentage was studied as affected by both type of glass ionomers and type of bleaching regardless of the time, the result was found to be highly significant (p < 0.01) [Table/Fig-2]. OX increased dissolution percentage of Photac Fil from 32.37±5.3 to 35.82±5.7, and it also increased that of F2000 from 34.48±4.9 to 38.16±3.7 [Table/Fig-3].
Dissolution percentage of glass ionomer materials related to type of bleaching regardless time.
B0= control samples. B1=samples bleached with opalescence xtra. B2=samples bleached with opalescence quick

Mean and standard deviation (n=90) of dissolution percentage of glass ionomer materials according to the bleaching types.
| Mean ±SD | Bleaching | Material |
|---|
| 34.57 ±4.87 | B0 | Ketac Fil |
| 29.18 ±3.89 | B1 | G1 |
| 32.44 ±4.26 | B2 | |
| 32.38 ±5.98 | B0 | Photac Fil |
| 35.82 ±5.74 | B1 | G2 |
| 33.81 ±3.27 | B2 | |
| 34.49 ±4.98 | B0 | F2000 |
| 38.16 ±3.73 | B1 | G3 |
| 35.48 ±3.36 | B2 | |
B0: a control group - B1: Bleached with Opalescence Xtra (OX) - B2: Bleached with Opalescence Quick (OQ)
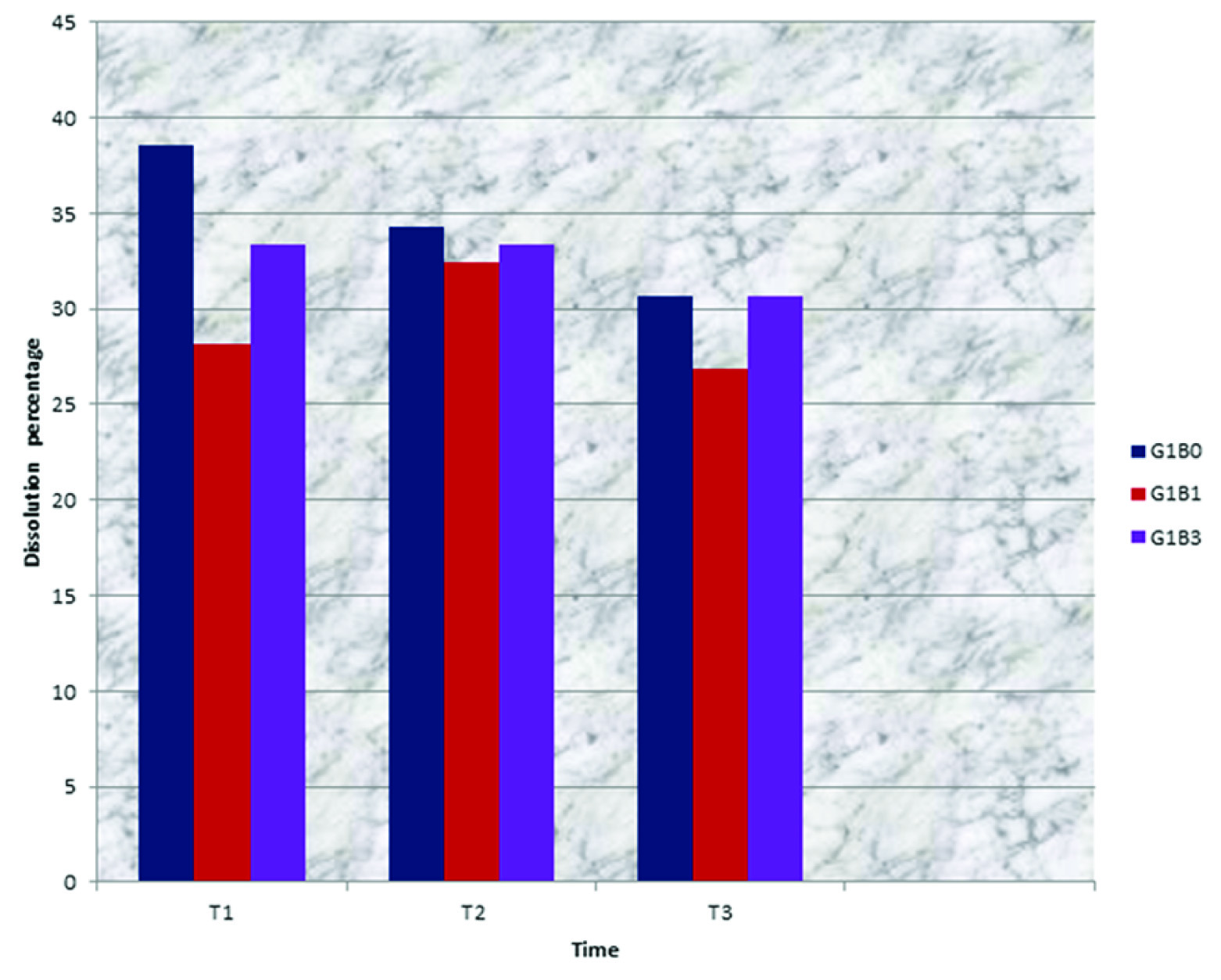
Bleaching with either OX or OQ did not affect dissolution percentage of Ketac Fil through all periods of the test [Table/Fig-4].
Dissolution percentage of glass ionomer materials related to time and type of bleaching for Ketac Fil
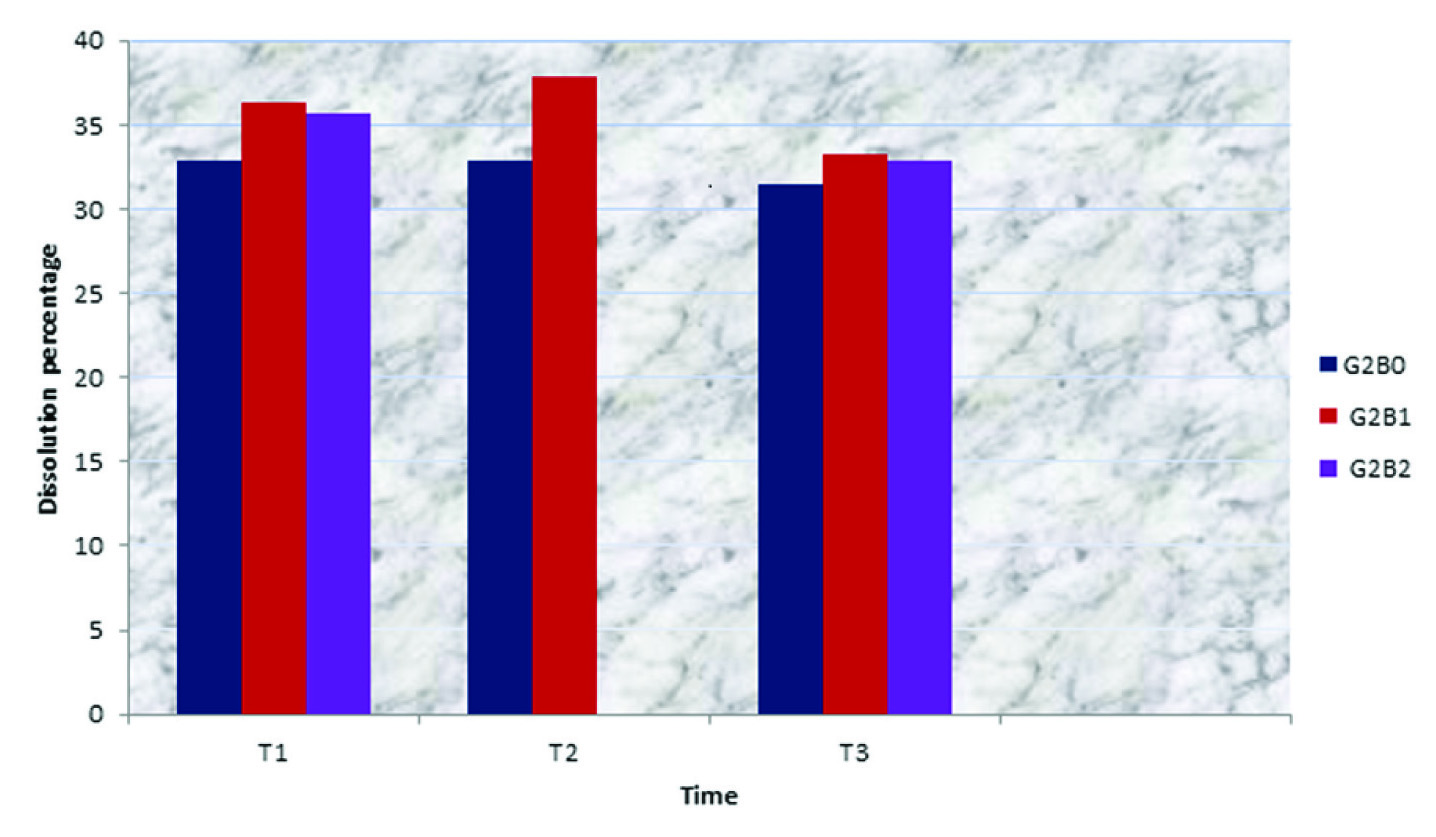
Increased dissolution percentage of Photac Fil which was bleached with OX was more prominent in the first and second periods of the test (T1 and T2) but it was not clear in the third period (T3) [Table/Fig-5].
Dissolution percentage of glass ionomer materials related to time and type of bleaching for Photac Fil
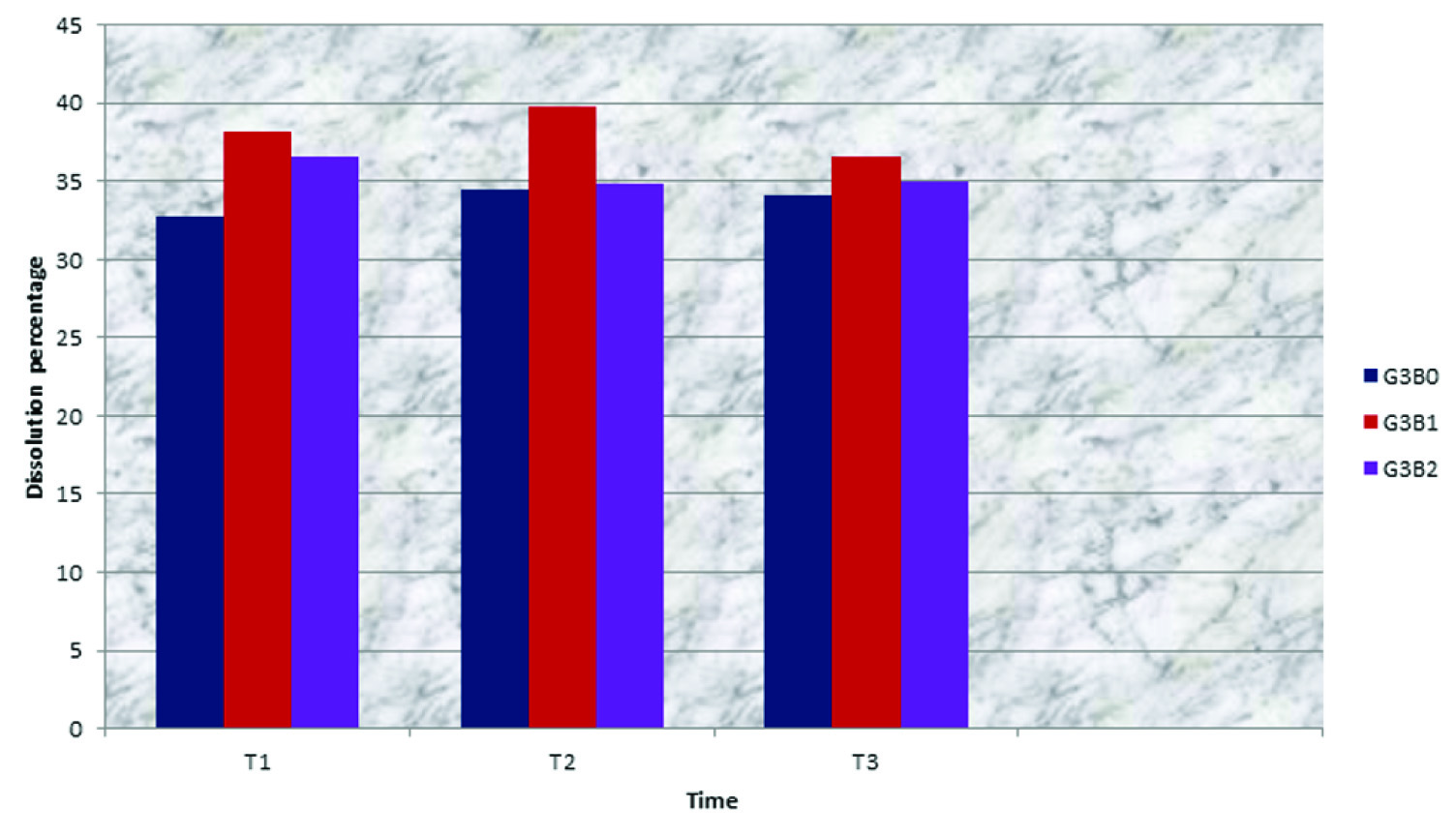
Dissolution percentage of F2000 which was bleached with OX increased through all periods of test, T1, T2 and T3. F2000 marked a more dissolution percentage than all the materials when they were bleached with OX [Table/Fig-6].
Dissolution percentage of glass ionomer materials related to time and type of bleaching for F2000
Opalescence Xtra (OX) showed a significant effect in increasing the dissolutions of both Photac Fil and F2000. Opalescence Quick (OQ) showed no significant effect on dissolutions of all materials which were under test.
Discussion
Since the introduction of bleaching by Haywood and Heymann 1989, the use of bleaching agents has become increasingly popular for whitening stained teeth. These products contact tooth structures for extended periods of time, especially during at-home bleaching treatments and they are unavoidable in prevention of restorations from exposure to bleaching agents [11].
Glass ionomers are often used for restoration of cervical lesions because of their bonding to tooth structures and as they release fluoride. At this site, the material is likely to be in contact with tooth whitening products. Bleaching therapies which use hydrogen peroxide or hydrogen peroxide releasing preparations may have a negative effect on restorations and restorative materials, as has been shown in numerous in vitro investigations [12].
In our study; the effect of bleaching on dissolution of glass ionomer restorations was material dependent; depending on the type of bleaching material and type of glass ionomer. Moreover, time factor was found to have an essential role.
The same conclusion was reached by Cehreli et al., who suggested that the effects of the bleaching gels appeared to be material-dependent when they evaluated the effects of two home-use bleaching gels on the surface roughness of different types of glass ionomers [13].
The insignificant effect of Opalescence Quick on dissolution of glass ionomers may be attributed to its lower concentration than that of Opalescence Xtra. It can also be explained by its more complicated steps of break down to free radicals and its slower rate of reaction than hydrogen peroxide, especially at room temperature and oral temperatures.
Mair and Joiner, classified the degradation of glass ionomers after bleaching to permanent; as a result of rupture of primary bonds in the material or reversible; as a result of softening and plasticizing of the surface. This is because glass ionomers consist of filler particles which are surrounded by a hydrogel matrix. The filler particles are designed to dissolve in acids, to enable the setting reaction to occur. Furthermore, the hydrogel matrix is permeable, which allows the bleaching agent access to sub surface of the material [14].
Turker and Biskin, stated that the pH of bleaching agents would affect the erosion rates of glass ionomers [15].
Some authors measured the pH of 26 tooth whitening products which were available in the market. They found that at home bleaching products had a pH range of 5.7 to 7.3. The pH of the in-office bleaching system was lower and it ranged from 3.6 to 6.5 [16]. It is highly probable that the pH of bleaching agents will affect the erosion rates of glass ionomers [15,17,18].
In our study, both bleaching materials were near to neutral (pH 7 for OX and 6.5 for OQ). Our results were in agreement with those of Taher, who concluded in 2005, that both in office and at home bleaching agents had a softening effect on some tooth coloured restorative materials [19]. Our results were also in agreement with those of Li et al., who concluded in 2009, that surface dissolution was detected in the polyacid-modified composite and glass ionomer cement when they were bleached with a 15% carbamide peroxide home bleaching gel [20]. The same idea was discussed by Lee et al., who concluded that highly concentrated bleaching regimes induced surface degradation, softening and an increase in fluoride release and changes in the coefficient of thermal expansion of polyacid-modified resin-based composites (compomers) when those bleaching agents were continuously applied for 1–5 days. In some products, even cracks were observed on the surfaces of the specimens [21].
Our results Contradicted with those of Miral and Joiner, who concluded in 2004, that a 6% hydrogen peroxide solution and whitening gel did not cause significant dissolutions of three glass ionomer materials (Chemeflex, Fuji II And Ketac Fill). This contrast in the results may be attributed to less concentration of bleaching (6%) which they used, than that in our study (38% hydrogen peroxide) [14]. Our study also showed that all glass ionomer restorative formulations exhibited a degree of dissolution.
Conclusion
The post-operative bleaching with 38% hydrogen peroxide increased dissolutions of both resin modified glass ionomers and compomers, while bleaching with 35% carbamide peroxide had no effect on dissolutions of glass ionomer restoratives.
T0=Weight of the samples before test (after 24 hours) - T1= Weight of the samples after one week - T2= Weight of the samples after one month - T3= Weight of the samples after 3 months
B0: a control group - B1: Bleached with Opalescence Xtra (OX) - B2: Bleached with Opalescence Quick (OQ)