Sirenomelia with Oesophageal Atresia: A Rare Association
Pragati Aditya Sathe1, Ratnaprabha Kundlikrao Ghodke2, Bhuvaneshwari Mahendra Kandalkar3
1Associate Professor, Department of Pathology,Seth G.S. Medical College & KEM Hospital, Mumbai, India.
2Associate Professor, Department of Pathology,Seth G.S. Medical College & KEM Hospital, Mumbai, India.
3 Professor, Department of Pathology, Seth G.S. Medical College & KEM Hospital, Mumbai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Pragati Aditya Sathe, A/7, Jeevan Sudha Society, C.D. Barfiwala Road, Andheri West, Mumbai-400058, India.
Phone: 9324045123 pragativk@yahoo.com
We are reporting a rare case of sirenomelia with oesophageal atresia. Sirenomelia is a lethal sporadic defect of which lower gastrointestinal tract anomalies are characteristic findings. Respiratory and upper gastrointestinal tract malformations like oesophageal atresia occur in about 20-35% of cases. Though its occurrence has been described, it has been reported only rarely. This report aims at describing this uncommon association along with its histological features.
Oesophageal atresia, Sirenomelia, Tracheoesophageal fistula, Mermaid
Introduction
Sirenomelia is a lethal condition which is characterized by fusion of lower limbs, single umbilical artery and severe musculoskeletal, urogenital and lower gastrointestinal tract malformations [1]. Tracheoesophageal anomalies are uncommonly reported with this condition [2]. This is a rare case of sirenomelia with oesophageal atresia and tracheoesophageal fistula.
Case Report
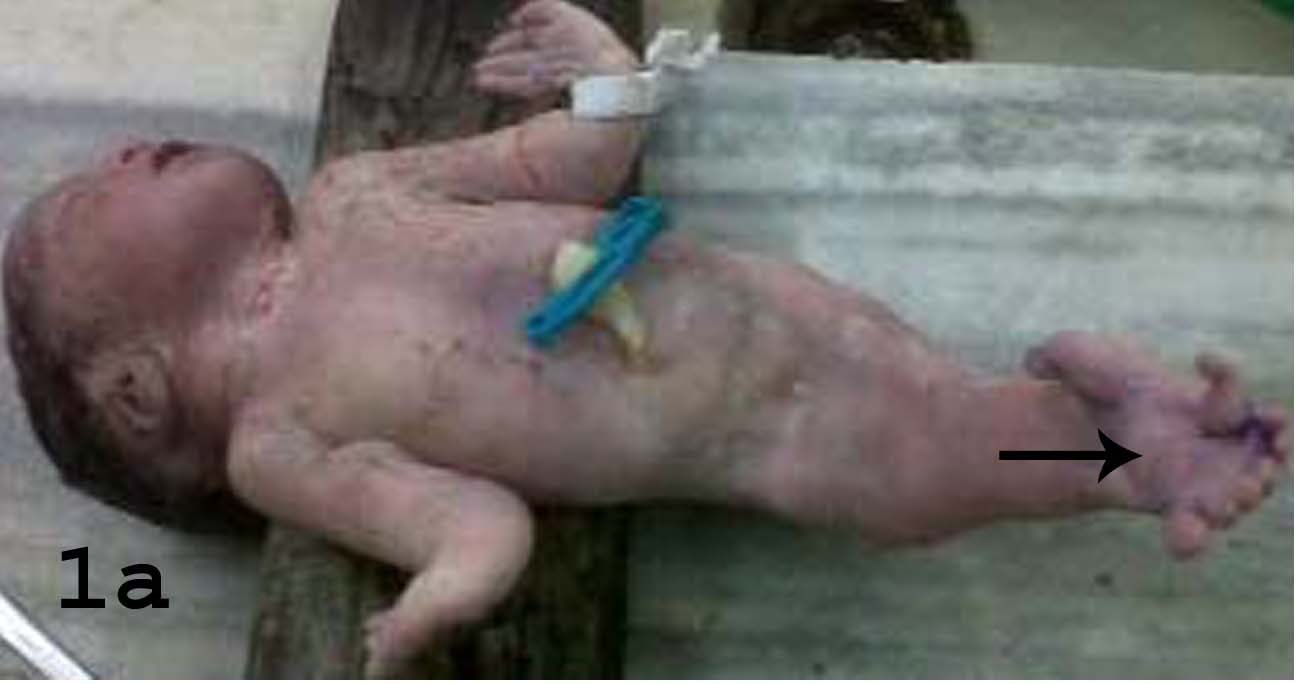
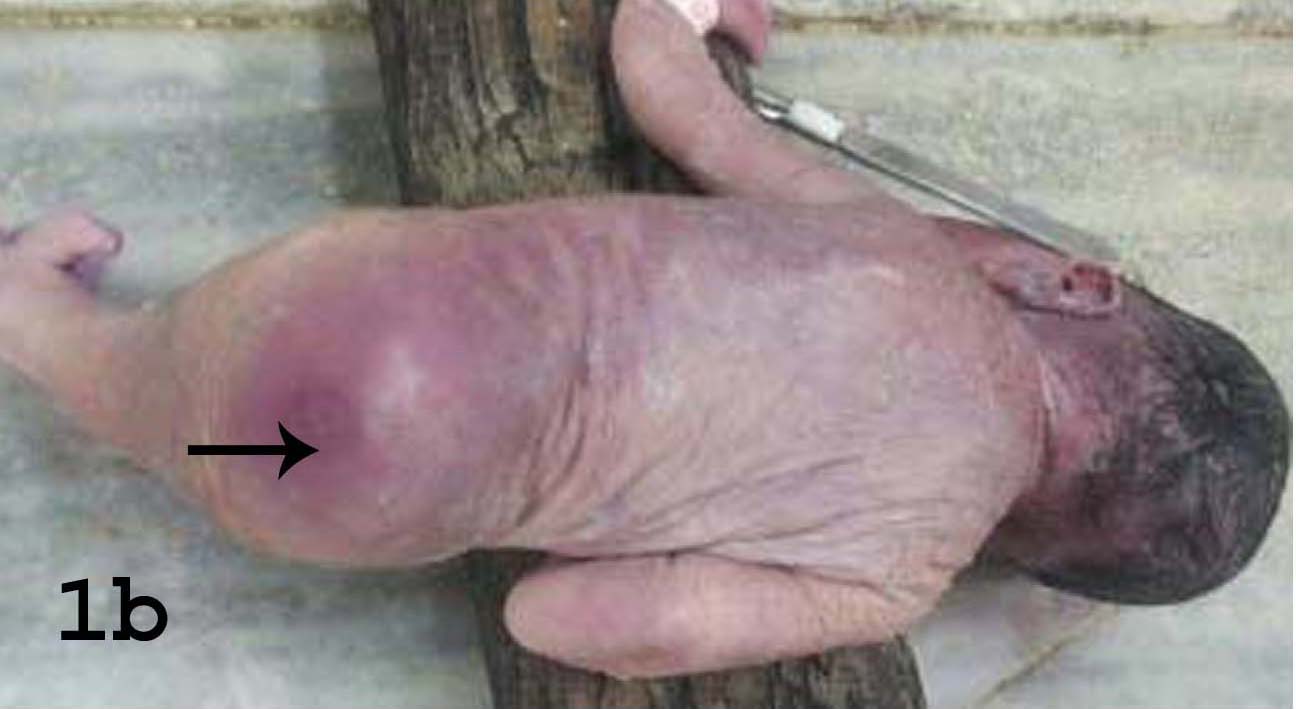
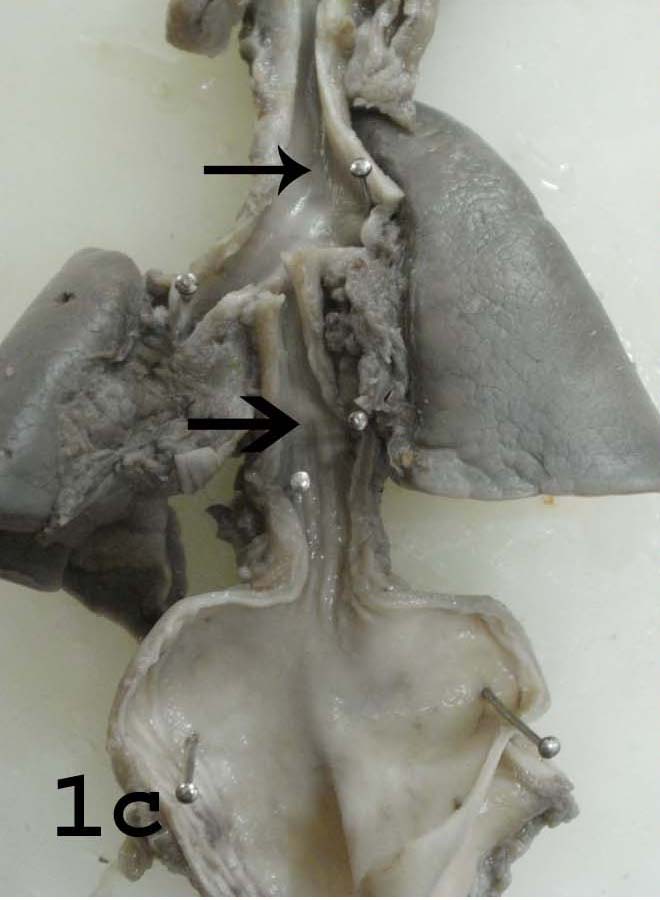
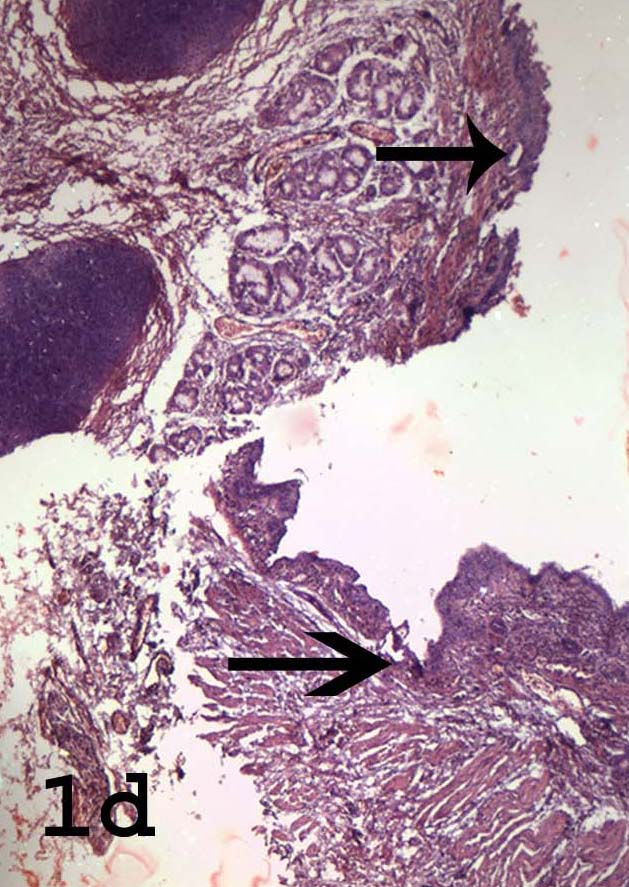
A 34-week, 1,500 gm, preterm infant, second by birth order, was born to a primigravida mother, by an elective lower segment caesarean section. Prenatal ultrasonography showed multiple congenital malformations, a ventricular septal defect, periventricular calcification and oligohydramnios. There was no history of maternal diabetes. The elder sibling of the patient was a still birth, the cause of which was unknown. No other family history of congenital anomalies or consanguinity was elicited. At birth, baby had a weak cry. After 20 minutes of birth, heart rate was 40 per minutes, but there was no spontaneous respiration. Despite all assisted measures, there was no improvement in heart rate or respiration and the child could not be revived. Autopsy was requested in view of congenital anomalies. At autopsy, pallor and cyanosis were noted. The lower limbs were fused from hip to toes and they showed eight appendages [Table/Fig-1a]. There was an imperforate anus, ambiguous external genitalia and urethral opening was absent [Table/Fig-1b]. Wrist showed plantar flexion deformity[Table/Fig-1a]. No obvious vertebral or facial deformities were noted. Detailed internal examination showed anomalies of various organ systems. Proximal oesophageal atresia was noted. The lower oesophageal segment communicated with the trachea at the carina (Type I tracheoesophageal fistula) [Table/Fig-1c]. The histology at the fistula demonstrated pseudo stratified columnar ciliated epithelium of the trachea and bronchi in continuation with squamous epithelium of oesophagus [Table/Fig-1d] .The lungs were largely non-expanded, as there was no spontaneous respiration. The heart showed a large patent foramen ovale. There was anal atresia. The large intestine was distended with meconium. A duplication cyst which was seen close to the blind end, showed an intestinal mucosal lining with all four layers and nerve plexuses within the wall. There was bilateral renal agenesis with absence of both ureters and the urinary bladder. A single testis was identified intra-abdominally, which showed the histology of an immature testis. The umbilical cord showed a single umbilical artery. The brain, liver, pancreas and adrenal glands did not show any abnormalities. There is an extra space between ‘was’ and ‘caused’.
Plantar flexion deformity of the wrist and fused lower extremities with eight appendages (arrow).
Gluteal region of the baby showing imperforate anus (arrow).
Tracheoesophageal fistula showing the lower oesophageal segment (lower arrow) communicating with the trachea (upper arrow) at carina.
Histology of the tracheoesophageal fistula showing the pseudo stratified columnar epithelium of the trachea (upper arrow) and squamous epithelium of lower oesophageal segment (lower arrow). (Haematoxylin-Eosin X 40)
Discussion
Sirenomelia, a sporadic defect, occurs in about 1 in 60,000 newborns. It is the result of a severe developmental defect of posterior axis caudal blastema [3]. The fused lower limbs show the appearance of “mermaid’s tail”; so the disease is also known as the mermaid syndrome.
The typical malformation of the lower limbs which is seen in babies with sirenomelia, consists of apparent fusion of the lower limbs. This can occur with varying severity, ranging from fusion of only the skin of lower limbs to the most severe form, which has only one lower limb tapering to a point, with the absence of foot structures [3].
Most of the sirenomelia cases result in stillbirths [1-3]. Though few survivors of this condition have been reported, such children rarely survive beyond the neonatal period [4]. The risk factors for sirenomelia include maternal diabetes, teratogens, genetic factors and maternal age which is less than 20 years [5].
The causes of sirenomelia are not very clear, but the abnormalities point to an insult which occurs at fourth week of gestation [5]. Abnormally formed umbilical cord blood vessels have been implicated. A normal foetus has two umbilical arteries which pump blood from the foetus to the placenta, and one umbilical vein, which returns blood from the placenta to the foetus. In the pelvis, the umbilical arteries branch off the iliac arteries, which supply the legs and pelvic organs, such as the genitalia.The “vascular steal theory” indicates that a single large artery assumes the function of the umbilical arteries, thus diverting blood flow from the caudal portion of the embryo to the placenta. The vitelline umbilical artery steals blood and nutrition from the lower body and diverts it to the placenta. This results in a small aorta and variable absence of the arteries that supply the kidneys, large intestine, and genitalia, resulting in maldevelopment of terminal bowel, kidneys, bladder, genital organs and pelvic bones [2,5]. Reports on sirenomelia with normal umbilical arteries point to the alternative caudal dysgenesis (CD) theory [5].
Cardiovascular, respiratory and upper gastrointestinal tract malformations occur in 20-35% of cases. Radial agenesis, oesophageal atresia and tracheoesophageal fistula, which are seen in some cases, suggest a VATER association and they may represent a lesser degree of caudal regression syndrome [3]. This is particularly seen in children of diabetic mothers. However, no such correlation was found in our case. Controversy exists in the literature regarding as to whether sirenomelia occurs as a separate entity or as the extreme form of caudal regression syndrome (CRS) [1-5]. However, the presence of two umbilical arteries, non-lethal renal anomalies, non-fused lower limbs, abdominal wall defects and abnormalities of tracheoesophageal tree, neural tube and heart, differentiates CRS from sirenomelia [1].
Because sirenomelia is a lethal condition, making an early antenatal diagnosis allows counselling for a possible pregnancy termination. In the setting of oligohydramnios and bilateral renal agenesis, the fusion of lower extremity clinches the antenatal diagnosis of sirenomelia [1].
Though the occurrence of sirenomelia with oesophageal atresia and tracheoesophageal fistula has been described, such cases have been reported only rarely. We found only one report which has described this association [2]. Also, the histological features of tracheoesophageal fistulae have rarely been studied.
[1]. BB Das, BK Rajegowda, R Bainbridge, PF Giampietro, Caudal regression syndrome versus sirenomelia: a case reportJ Perinatol 2002 22:168-70. [Google Scholar]
[2]. S Sozubir, F Guven, T Ozkamaci, Sirenomelia with oesophageal atresia AdvClin Path 2000 4:165-8. [Google Scholar]
[3]. JM Opitz, GN Wilson, E Gilbert-Barness, Analysis of developmental pathology. In: Gilbert-Barness E, edPotter’s Pathology of the Fetus, Infant and Child 2007 PhiladelphiaMehta Publishers:97-133. [Google Scholar]
[4]. MP Stanton, EC Penington, JM Hutson, A surviving infant with sirenomelia (Mermaid syndrome) associated with absent bladderJ Pediatr Surg 2003 38:1266-8. [Google Scholar]
[5]. A Fadhlaoui, M Khrouf, S Gaigi, F Zhioua, A Chaker, The sirenomelia sequence: a case historyClin Med Insights Case Rep 2010 3:41-9. [Google Scholar]