CaCx is one of the most common cancer in women, and is responsible for highest number of cancer deaths among young females in developing countries [1]. CaCx is considered as a preventable disease because it is preceded by pre-invasive stages. The conventional Papanicolaou (Pap) smear examination, though useful, yields false negative result in approximately 20-30% cases [2,3]. It has been observed that certain high risk genotypes of HPV are constantly associated with 99.7% of SCC cases worldwide [4]. Of these high risk genotypes, HPV 16 and 18 were observed to be responsible for approximately 62% cases of CaCx from around the globe [5]. These findings indicate the significance of HPV detection in cervical cytology samples in screening of CaCx.
Further, the need of HPV testing was also highlighted by the finding of high grade intraepithelial lesions in 83% of women with normal cytology and positive HPV mRNA test [6]. Several kits, which are based on sequences or signal amplification and/or detection by hybridization, got Food and Drug Administration (FDA) approval for detection of high risk HPV specific Deoxyribonucleic acid (DNA) or E6 mRNA in cervical samples [7]. NMPCR targeting E6/7 gene has got the ability to detect 13 heart rate (HR)–HPV and 2 low risk HPVs with a potential to detect target gene sequence of viral DNA in order of 1 fg (102 viral copy) [8]. It appears to be cost effective as it obviates the need of hybridization with specific probes.
In the present study, we investigated the utility of NMPCR based detection of HPV 16 and 18 in pre-invasive lesions of CaCx and its implications in screening of CaCx in conjunction with Pap smear examination.
Materials and Methods
A total of 119 females, in the age group of 20 to 70 years, attending as outpatients in Department of Obstetrics and Gynaecology, University Hospital, Banaras Hindu University, Banaras, UP, India, were enrolled in this hospital based prospective study which spanned for a period of one year from September 2009 to August 2010. Informed consent was obtained from each patient included in the study. Cervical scrapes were collected with the help of Ayer’s spatula. Scrapings were first spread on sterile grease free slide and fixed for modified Pap staining and the remaining part of the scrapings was dipped in sterile phosphate buffer saline (PBS) maintained at pH 7.4 for DNA based study. Cell pellet obtained after centrifugation of PBS solution were re-suspended in 200 μl EDTA buffer and preserved at 4°C till further processing.
Punch biopsies were collected from the patients presenting with growth over cervix suggestive of neoplasia or reported with high grade dysplastic changes on Pap smear examination. The tissue samples were divided and kept in two separate vials containing 10% formalin solution and PBS for histological examination and DNA isolation respectively. The formalin fixed biopsy specimens were examined and paraffin embedded blocks were prepared and further subjected to routine Haematoxylin and Eosin (H&E) staining for light microscopic studies as per standard protocol [9]. Histopathological examination of the Pap smears and punch biopsy samples were performed. All the Pap smears were examined by two independent observers and graded according to Bethesda Classification 2001.
NMPCR targeting E6/E7 gene sequence of HPV 16 and 18 was performed on cervical scrapes and/ or punch biopsy samples employing the primers as described [Table/Fig-1] [8]. DNA extraction was done from cells harvested from clinical samples by using the phenol-chloroform method [10], with few modifications. Standardization of nested PCR was done using DNA isolated from SiHa and HeLa 229 cell lines as positive controls for HPV 16 and 18 respectively. Optimization of annealing temperature for first and nested cycles for detection of HPV 16 and 18 specific E6/7 gene sequences, integrated in these cell lines, was attempted in gradient thermal cycler (Bio-Rad, USA).
Primers for amplification of HPV 16 and 18 specific E6/7 gene sequence. Sotlar et al; J Clin Microbiol 2004; 42:3176-84 [8]
| Sl No | Code | Oligo sequence | Expected size of amplicons |
|---|
| 1 | GP-E6-3F | 5’- GGG WGK KAC TGA AAT CGG T -3’ | 630 bp |
| 2 | GP-E7-5B | 5’-CTG AGC TGT CAR NTA ATT GCT CA-3’ |
| 3 | GP-E7-6B | 5’-TCC TCT GAG TYG YCT AAT TGC TC-3’ |
| 4 | 16 F | 5’-CAC AGT TAT GCA CAG AGC TGC-3’ | 457 bp |
| 5 | 16 R | 5’-CAT ATA TTC ATG CAA TGT AGG TGT A-3’ |
| 6 | 18 F | 5’-CAC TTC ACT GCA AGA CAT AGA-3’ | 322 bp |
| 7 | 18 R | 5’-GTT GTG AAA TCG TCG TTT TTC A-3’ |
NMPCR was executed on DNA isolated from the cervical scrapes and biopsy along with positive controls for HPV 16 and 18 as mentioned above and double distilled water as negative control. Briefly, the reaction mixture for the first round PCR contained 2.5 μl of 10× PCR buffer (GeNei, Bangalore, India), 10 pmol of each primer GP-E6-3F, GP-E7-5B and GP-E7-6B (IDT, USA), 1 μl of deoxynucleoside triphosphate mix (GeNei), 1 U of Taq DNA polymerase (GeNei), 10 μl of DNA template, and water to a final volume of 25 μl. The first-round amplification was carried out in a thermocycler (Biometra, Goettingen, Germany) under the following conditions: 40 cycles for 1 minute and denaturation at 94°C, 1 minute annealing at 44°C, and 1 minute elongation at 72°C, with a final elongation step extended to 7 minutes.
The nested PCR master mix was the same as that of the first-round PCR, except it contained 15 pmol of each primer 16 F, 16R, 18F, and 18 R and 5 μl of DNA template (1:5 diluted product of the primary cycle). Thermal cycling was carried out as described for first round PCR, except that the annealing temperature was set to 56°C. To separate amplified products, 5 μl of solution was electrophoresed on a 1.5% agarose gel in TBE (Tris-borate-EDTA) buffer at 80 V for 1 h. The gels were stained with ethidium bromide, and the bands were visualized under UV light.
The Chi-square test was applied to calculate the significance of association of HPV 16/ 18 positivity with different pre-invasive lesions of CaCx. The study was approved by the Institute Ethics Committee of the university.
Results
Present study is based on observations made on 105 cervical smears and 20 cervical biopsies obtained from 119 subjects enrolled for the study. These patients ranged between 21 and 66. On physical examination cervix was found unhealthy in 83 patients (Group A) while in 36 subjects it was apparently healthy (Group B).
On cytological examination 72 smears were categorized as Negative for Intraepithelial Lesion or Malignancy (NILM), 2 as Atypical Cells of Undetermined Significance (ASCUS), 25 as Low grade Squamous Intraepithelial Lesion (LSIL), and six as High grade Squamous Intraepithelial Lesion (HSIL). Reactive cellular changes were also observed in 45 cases which were grouped as NILM [Table/Fig-2]. Of the 20 biopsies that were studied, 6 biopsies from the patients, were categorized as HSIL on cytological examination. Of these 6 cases 3 were reclassified as SCC on biopsy, two as CIN II/III and one as CIN I. Among the rest of the 14 biopsies, 12 were classified as SCC while other 2 were categorized as adeno CaCx [Table/Fig-3].
HPV 16/18 positivity correlation with cytological diagnosis
| Group | HPV positivity status | NILM | ASCUS | LSIL | HSIL |
|---|
| Group A | HPV 16 | 7/44 | 0/1 | 7/19 | 3/5 |
| 17/69 |
| HPV 18 | 3/44 | 0/1 | 1/19 | 0/5 |
| 4/69 |
| Group B | HPV 16 | 3/28 | 0/1 | 2/6 | 0/1 |
| 5/36 |
| HPV 18 | 2/28 | 0/1 | 1/6 | 1/1 |
| 4/36 |
| Total HPV 16/ 18 positivity | 30/ 105 | 15/72 | 0/1 | 11/25 | 4/ 6 |
Group A: Subjects with unhealthy cervix, Group B: Subjects with healthy cervix; NILM: Negative for intraepithelial lesion or malignancy, ASCUS: Atypical squamous cell undetermined significance, LSIL : Low grade squamous intraepithelial lesion, HSIL: High grade squamous intraepithelial lesion
HPV 16/18 positivity correlation with histological diagnosis
| Group | HPV status n=20 | CIN I n=1 | CIN II/ III n=2 | SCC n=15 | Adenocarcinoma n=2 |
|---|
| Group A | HPV 16 13/19 HPV 18 0/19 | 0/1 | 1/ 1 | 12/ 15 | 0/2 |
| 0/1 | 0/ 1 | 0/ 15 | 0/2 |
| Group B | HPV 16 0/1 HPV 18 1/1 | — | 0/ 1 | — | — |
| — | 1/ 1 | — | — |
Group A: Subjects with unhealthy cervix, CIN: Cervical Intraepithelial Neoplasia, SCC: Squamous cell carcinoma. Note: None of the subjects with healthy cervix (Group B) were subjected for biopsy. 15 cases of SCC includes, 3 cases which were initially reported as HSIL
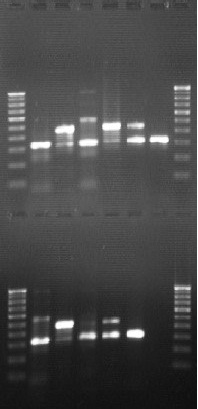
Standardisation of nested PCR for detection of HPV 16 and 18
Lane A1, A8, B1 and B8: 100 bp DNA ladder
Lane: A2, 4, 6, B2, 4, 6: HeLa cell line (322 bp)
Lane: A3, 5, 7, B3, 5, 7: SiHa Cell line (457 bp)
(Lane A 1-8: Above; Lane B 1-8: Below)
The mean age of the patients harbouring LSIL and HSIL was 39 and 40 respectively. The mean age of the patients diagnosed as SCC was 49 years while the patients diagnosed as adenocarcinoma was 61.
On standardization of Nested PCR, targeting integrated HPV oncogene E6/ E7 in SiHa and HeLa cell lines, for detection of HPV 16 (457 bp) (Lane B 3) and HPV 18 specific (322 bp) sequence (Lane B 2) respectively, the optimum annealing temperature for first and second round of PCR was observed to be 44 and 56°C respectively [Table/Fig-4]. HR-HPV was detected in 30 cytology specimens and in 13 biopsy specimens and these findings have been shown in [Table/Fig-2,3].
Of the 72 cases reported as NILM on Pap smear examination, 44 cases were from Group A and 28 cases from Group B. HR-HPV was detected in 20.8% (15/72) cases categorized as NILM. Further, 22.7% of NILM cases in Group A and 17.8% of Group B were found to be positive for these HPVs. Eighty percent (12/ 15) HPV positive NILM cases were those which also showed reactive cellular changes on cytology smear examination [Table/Fig-5].
HPV 16/18 positivity among different subcategories of NILM
| HPV status | NILM# n=72 | Reactive cellular changes n=45 | WNL$ n=27 |
|---|
| HPV 16/18 Positivity | 15 (20.8) | 12 (26.6) | 3 (11.1) |
Data in parenthesis indicates percentage
#NILM : Negative for intraepithelial lesion or malignancy
$WNL: Within Normal Limit
Out of 25 cases categorized as LSIL, 19 were in Group A, of which 42% were reported positive for HPV whereas 50% (3 of 6) cases presented with LSIL in Group B were HPV positive [Table/Fig-2]. Of the 6 HSIL lesions, 5 were from Group A and 60% of them were positive for HPV 16 whereas the only case detected positive for HPV with HSIL of Group B, was found to be positive for HPV 18.
Of the five cases categorized as HSIL in Group A, three cases were found to be suffering from SCC, one was found to have CIN III and positive for HPV 16, and one case was reported as CIN I and not detected positive for HPV 16/18. The single case of HSIL observed in Group B, positive for HPV 18, was found to harbour CIN II [Table/Fig-3]. Of the 15 cases of SCC, majority (80%) were observed to be associated with HPV 16 [Table/Fig-6]. None of the two cases, diagnosed as adenocarcinoma, were found positive for either of the genotypes of HPV.
PCR for detection of HPV 16 in carcinoma cervix
Lane 1: 100 bp DNA ladder, Lane 2: SiHa cell line
Lane 4-8: HPV 16 positive cases of Carcinoma cervix (457 bp)
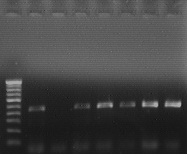
On analyzing the data statistically, no significant association was observed between pre invasive lesions HSIL, and LSIL and unhealthy cervix (p>0.05). Association of HPV 16 in cervical scrapes of females with unhealthy cervix was found to be significantly higher (p<0.05). However, the HPV 16/18 positivity in both the groups was statistically comparable (p> 0.05).
Discussion
HPV on the basis of their association with CaCx has been classified into low risk, intermediate and high risk genotypes. Apart from HPV 16 and 18, 13 other mucosotropic HPV genotypes viz. 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82 have been considered carcinogenic till date [11]. Globally, about 11.4 % women in the general population are estimated to harbour cervical HPV infection. It has been observed that worldwide prevalence of HPV 16 and or 18 among women with normal cytology, LSIL/CIN-1, HSIL/ CIN-2/CIN-3/CIS, and CaCx observed to be 3.8, 24.3, 51.1, 70.9 % respectively whereas in India the respective percentage distribution observed was 6.0, 29.4, 56.0, and 82.5 [12].
It has been observed that the treatment for pre-invasive disease is effective and thus screening can potentially prevent the occurrence of invasive CaCx. The requirement of an effective screening programme was also felt when impaired cell mediated immunity emerged as one of the risk factors for HPV induced carcinogenesis of lower genital tract [13]. In the present study, although the percentage positivity of pre invasive lesions HSIL, and LSIL observed to be more in females who presented with unhealthy cervix than those with healthy cervix, its association was not significant (p>0.05). This emphasizes the importance of screening of CaCx in all eligible females irrespective of physical status of cervix on clinical examination.
As the population at risk of CaCx is high in developing countries, it is important to use a highly sensitive, specific and cost-effective molecular tool to screen for HR-HPV. Recently, a clinical trial conducted on rural population in India, suggested the usefulness of single HPV testing in reducing the mortality in significant way [14]. In this study, it was observed that both HPV 16 and 18 could be detected in samples of both the groups, but HPV 16 positivity was significantly higher in females presenting with unhealthy cervix (p<0.05). Moreover, on comparing the HPV 16/18 positivity among the members of both Groups A and B, no significant difference was observed (p>0.05); suggesting the importance of HPV testing in all the eligible females irrespective of condition of cervix.
Further, it was observed that of all the cervical scrapes HPV 16 positivity was 36% among LSIL, 50% of HSIL, and 80% of SCC; whereas HPV 18 was found to be associated with 8% of LSIL, and 16.7% HSIL. In a similar study, reported from Japan, HPV 16 was detected in 17.6% and 35% cases of LSIL and HSIL respectively whereas HPV 18 in 8.8% and 8.3% of LSIL and HSIL respectively indicating that the percentage distribution of these genotypes differ in different geographic location [15].
In the present study, 20.8% cases of NILM cases were found to be HPV 16/ 18 positive. Of these, 13.9% were positive for HPV 16 whereas HPV 18 was positive in 6.9%. In India HPV 16 and 18 positivity among subjects with normal cytology was 4.7% and 1.3% respectively [10]. Apart from cases reported with normal cytology, NILM cases also included 45 cases with reactive cellular changes. In such cervical Pap smears, excess of inflammatory cells may obscure the interpretation of cellular changes associated with HPV infections [16]. Similarly, excess of non target leukocyte DNA, present in the DNA template, isolated from cervical scrapes may act as relative PCR inhibitor [17]. In an earlier study, HPV infection was detected in 22.6 percent of cervical biopsy specimens of women whose cervical smears showed reactive cellular changes [18]. In present study, 26.6% cases with NILM, subcategory reactive cellular changes, turned out to be positive for HPV 16/ 18. This high positivity for HPV 16/ 18 among these cases might be due to higher sensitivity of nested PCR protocol, which has the ability to detect fewer target DNA even in the presence of relatively excess of non target human DNA and thus obviates the need for biopsy samples for the detection of HPV.
The advantage of PCR protocol employed in the present study is that it can be standardised in house, using Hela and SiHa cell lines as positive controls, for detection of 2 most common HR-HPV genotypes associated with SCC of uterine cervix. The inherent limitation of the protocol is its inability to detect other HR-HPVs associated with 20% of cases of SCC which were found to be negative for HPV 16/18. Hence, there is a need for change in testing protocol to investigate the occurrences of other HR-HPV genotypes in this region, i.e., eastern part of northern India.
Conclusion
Present study has demonstrated the usefulness of NMPCR detection of HPV 16 and 18 in cases reported with different stages of pre invasive lesions of CaCx, particularly in the subcategory reactive cellular changes of NILM. The protocol may be used as a screening tool to screen females at risk for CaCx along with Pap smear examination.
Authors’ Contributions
AKG, PP, CD, SS: Conceived the hypothesis and reviewed the literature; AKG, SP, MK: Designed the study plan; SP, LKP, CD: Recruited study population and collected samples; MK, AGK: Performed Cytological and Histopathological Examinations; PP, SS, GN: Performed PCR based studies; PP, AGK: Drafted manuscript; GN, AKG, LKP: Critically reviewed the manuscript.
Group A: Subjects with unhealthy cervix, Group B: Subjects with healthy cervix; NILM: Negative for intraepithelial lesion or malignancy, ASCUS: Atypical squamous cell undetermined significance, LSIL : Low grade squamous intraepithelial lesion, HSIL: High grade squamous intraepithelial lesion
Group A: Subjects with unhealthy cervix, CIN: Cervical Intraepithelial Neoplasia, SCC: Squamous cell carcinoma. Note: None of the subjects with healthy cervix (Group B) were subjected for biopsy. 15 cases of SCC includes, 3 cases which were initially reported as HSIL
Data in parenthesis indicates percentage#NILM : Negative for intraepithelial lesion or malignancy$WNL: Within Normal Limit