Relationship between Lipoprotein(a) and Thyroid Hormones in Hypothyroid Patients
Ramachandran Kaliaperumal1, Ebenezer William2, Thangapaneer Selvam3, Shyam M. Krishnan4
1 Tutor, Department of Biochemistry, SRM Medical College Hospital and Research Centre, Kattankulathur, Kanchipuram, Tamilnadu-603203, India.
2 Professor and Head, Department of Biochemistry, SRM Medical College Hospital and Research Centre, Kattankulathur, Kanchipuram, Tamilnadu-603203, India.
3 Professor, Department of Biochemistry, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research, Melmaruvathur, Tamilnadu-603319, India.
4 PostGraduate, Department of Biochemistry, SRM Medical College Hospital and Research Centre, Kattankulathur, Kanchipuram, Tamilnadu-603203, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ramachandran Kaliaperumal, Tutor, Department of Biochemistry, SRM Medical College Hospital and Research Centre, Kattankulathur, Kanchipuram, Tamilnadu-603203, India.
Phone: +91-9944959174,
E-mail: ramkalai26092012@gmail.com
Background and Objective: Changes in plasma lipid concentrations are well known metabolic consequences of thyroid dysfunction. The alterations are most prominent in hypothyroidism which is typically associated with pronounced hypercholesterolaemia and frequently with moderate hypertriglyceridaemia. In cases of hypothyroidism, how the serum Lp(a) levels are influenced by thyroid hormone remains unknown and contradictory results on the effect of thyroid hormone on serum Lp(a) levels have been reported. There is substantial evidence to suggest that elevated serum Lp(a) levels contribute significantly to the development of CHD. The present study was designed to determine the lipoprotein(a) [Lp(a)], lipid profile and thyroid hormone levels in newly diagnosed hypothyroid patients and to find any correlation that existed between Lp(a) and other parameters.
Materials and Methods: Untreated hypothyroid (n=50) patients were included in the study. We also included 40 normal healthy subjects as controls. Lipid profile, Lp(a) and thyroid profile were estimated by using autoanalyzers.
Results: The results of this study showed that levels of HDL-cholesterol were significantly decreased (p<0.001), whereas those of other lipid parameters and Lp(a) levels were found to be significantly increased (p<0.001) in hypothyroid patients as compared to those in controls. Correlation study revealed a significant positive correlation between Lp(a) and TSH levels in hypothyroid patients.
Conclusion: Our present findings indicated that hypothyroidism could be strongly associated with lipid abnormalities that enhanced the development of cardiovascular diseases. Also, Lp(a) and non-HDL-C should be estimated with other lipid parameters as a useful index for measuring the cardiac risk in hypothyroid patients. A recommended screening should be advised for any patient with thyroid dysfunction, especially hypothyroidism, to assess lipid abnormalities by using Lp(a) and non- HDL-C and he/she should treated at the earliest.
Cardiovascular disease, Non-HDL-C, Hyperlipidaemia
Introduction
Hypothyroidism is a common metabolic disorder which is existent in the general population. Levels of total and LDL cholesterol tend to increase as the thyroid function declines. Hypothyroidism is associated with an increased prevalence of coronary heart disease (CHD), presumably because of the hyperlipidaemia which is frequently associated with this disease, which is characterized in most cases by hypercholesterolaemia.
Much attention has been paid to the clinical significance of lipoprotein(a) [Lp(a)]. Lp(a) is an LDL-like particle which has been linked to a glycoprotein which has been named as apolipoprotein(a). There is substantial evidence to suggest that elevated serum Lp(a) levels contribute significantly to the development of CHD [1–5].
Nevertheless, the physiological role and metabolism of Lp(a) are not entirely clear. In cases of hypothyroidism, how the serum Lp(a) levels are influenced by thyroid hormone remains unknown and contradictory results on the effect of thyroid hormone replacement on serum Lp(a) levels have been reported [6–13]. Although an altered lipid profile is frequently observed in patients with thyroid dysfunction, published data on the relationship between Lp(a) and thyroid status are controversial [14].
In a number of previous studies, investigators have reported the effect of the thyroid status on the changes in the serum lipoprotein concentrations, whereas reports on serum Lp(a) concentrations are limited and the results are controversial. Recently, non-high-density lipoprotein cholesterol (non-HDL-C), a measure of Total Cholesterol (TC) minus HDL-C, has emerged as a predictor of cardiovascular disease. Elevated levels of low-density lipoprotein cholesterol (LDL-C) have been consistently associated with an increased risk for development of cardiovascular disease. The present study was done to determine the lipoprotein (a), lipid profile and non-HDL-C levels in hypothyroid patients and to find any significant correlation which existed between Lp(a) and non-HDL-C and thyroid hormones.
Materials and Methods
Study Design
The correlation analysis study design was performed on hypothyroid and healthy individuals. We recruited 50 newly diagnosed hypothyroid patients who had been referred to a reputed hospital and 40 healthy individuals who served as controls. Blood samples were collected in sterile plain tubes after an overnight fast and they were centrifuged at 3000 rpm for 10 minutes. The fresh serum samples were then analyzed for specific parameters which have been mentioned further.
Methods
The levels of TC, HDL-C, and triglycerides (TG) were measured by enzymatic assays. The non-HDL-C levels were calculated as TC-HDL-C. The LDL-C levels were measured by direct homogenous assay. The serum Lp(a) concentration was measured by using a latex turbidimetry method. All the above parameters were analyzed by using auto analyzer Olympus AU400. Serum concentrations of free T4 (reference range, 0.8 - 1.8 ng/dL), free T3 (2.1-4.2 pg/mL), and TSH (0.5 - 5.0 μIU/mL) were measured by two-site immunoenzymometric assay by using TOSOH AIA 360 system analyzer.
The study protocol was in accordance with the Helsinki Declaration of 1975, that was revised in 2000 and with the institutional ethics committee’s guidelines. Informed consents were obtained from all patients and healthy volunteers before they entered the study.
Statistical Analysis
The data were expressed as Mean ± SD for [Table/Fig-1] and as Mean ± SE for [Table/Fig-2]. Statistical analysis was performed by employing t-test. The correlation of parametric values was performed by using Pearson’s correlation analysis. p values which were ≤ 0.05 were considered to be statistically significant.
General parameters of both patients and controls.
| Parameters | Healthy Controls (n = 40) | Hypothyroid Patients (n = 50) | p Value |
|---|
| Age | 47±13.5 | 50.3±8.8 | 0.24 (NS) |
| BMI | 20.4±2.8 | 23.2±7.4 | 0.02* |
| Male/Female | 16/24 | 14/36 | |
*Correlation is significant at the 0.05 level (2-tailed).
NS: Not significant,**Correlation is significant at the 0.01 level (2-tailed)
Parameters of patients compared with controls
| Parameters | Controls | Patients | p Value |
|---|
| FT4 | 1.35±0.05 | 0.36±0.02 | 0.0001** |
| HDL - C | 41.1±0.78 | 30.54±0.88 | 0.0001** |
| LDL - C | 105.9±2.32 | 159.10±4.91 | 0.0001** |
| LDL/HDL - C | 2.62±0.08 | 5.09±0.23 | 0.0001** |
| Lp(a) | 18.65±0.72 | 27.02±0.58 | 0.0001** |
| non-HDL | 128.55±2.61 | 201.06±5.15 | 0.0001** |
| TOTAL CHOLESTEROL | 169.65±2.59 | 232.12±5.12 | 0.0001** |
| TRIGLYCERIDES | 112.95±4.49 | 219.34±9.16 | 0.0001** |
| TSH | 2.14±0.09 | 47.82±4.39 | 0.0001** |
| VLDL | 22.59±0.89 | 43.86±1.83 | 0.0001** |
*Correlation is significant at the 0.05 level (2-tailed)
NS: Not significant,**Correlation is significant at the 0.01 level (2-tailed)
Results
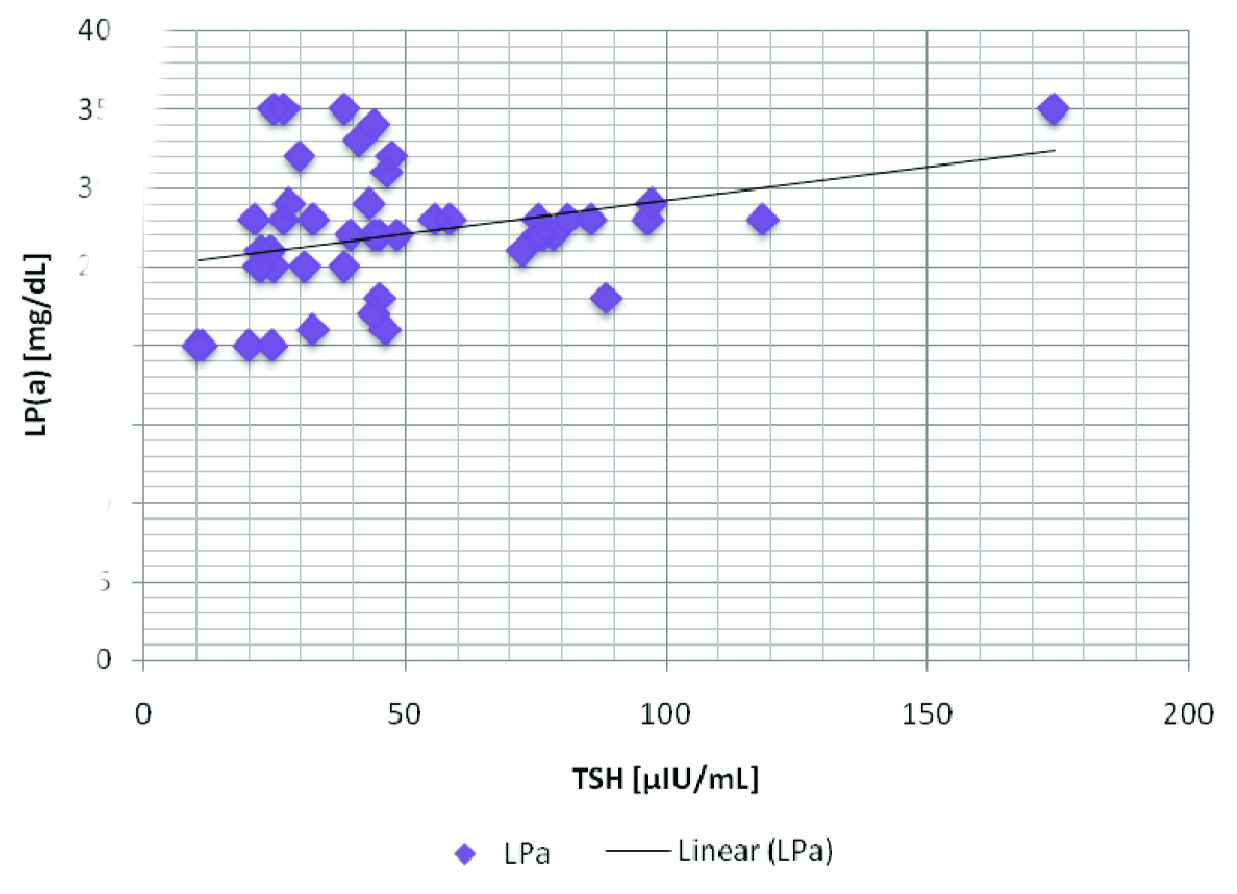
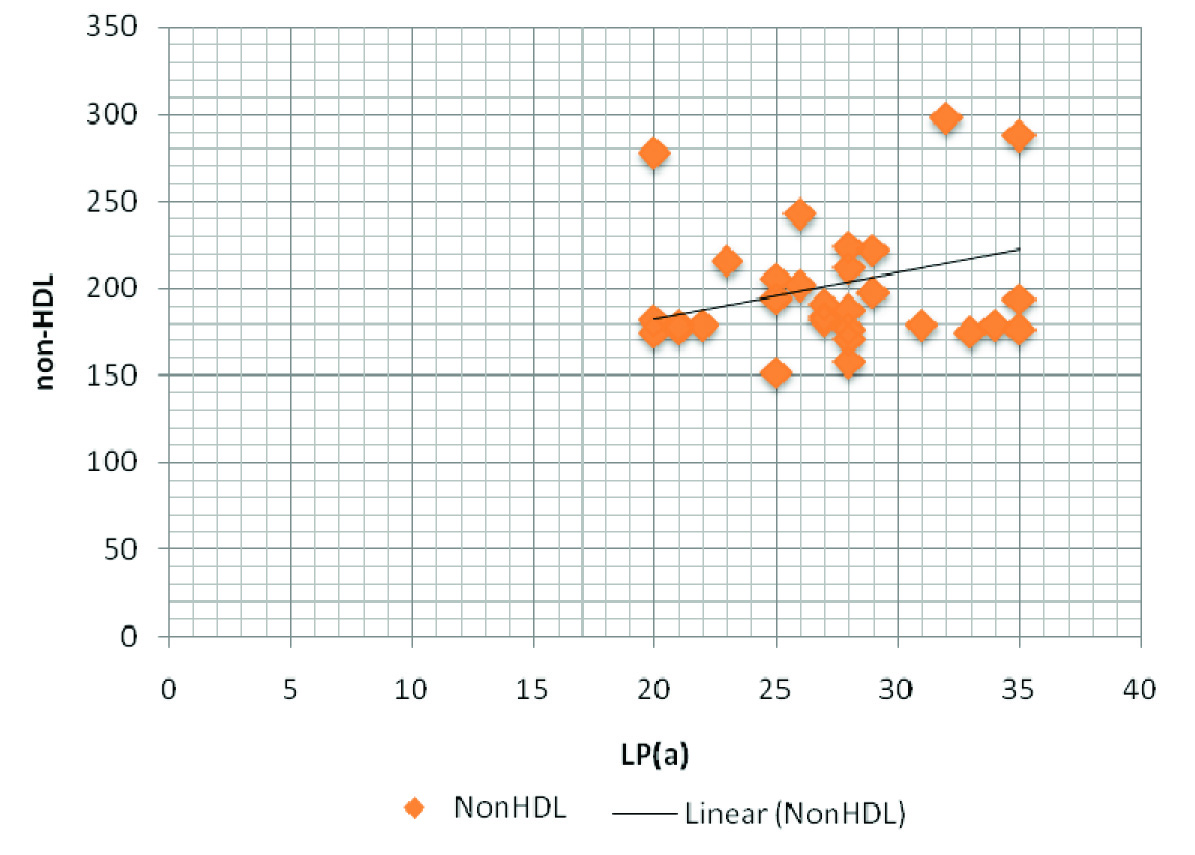
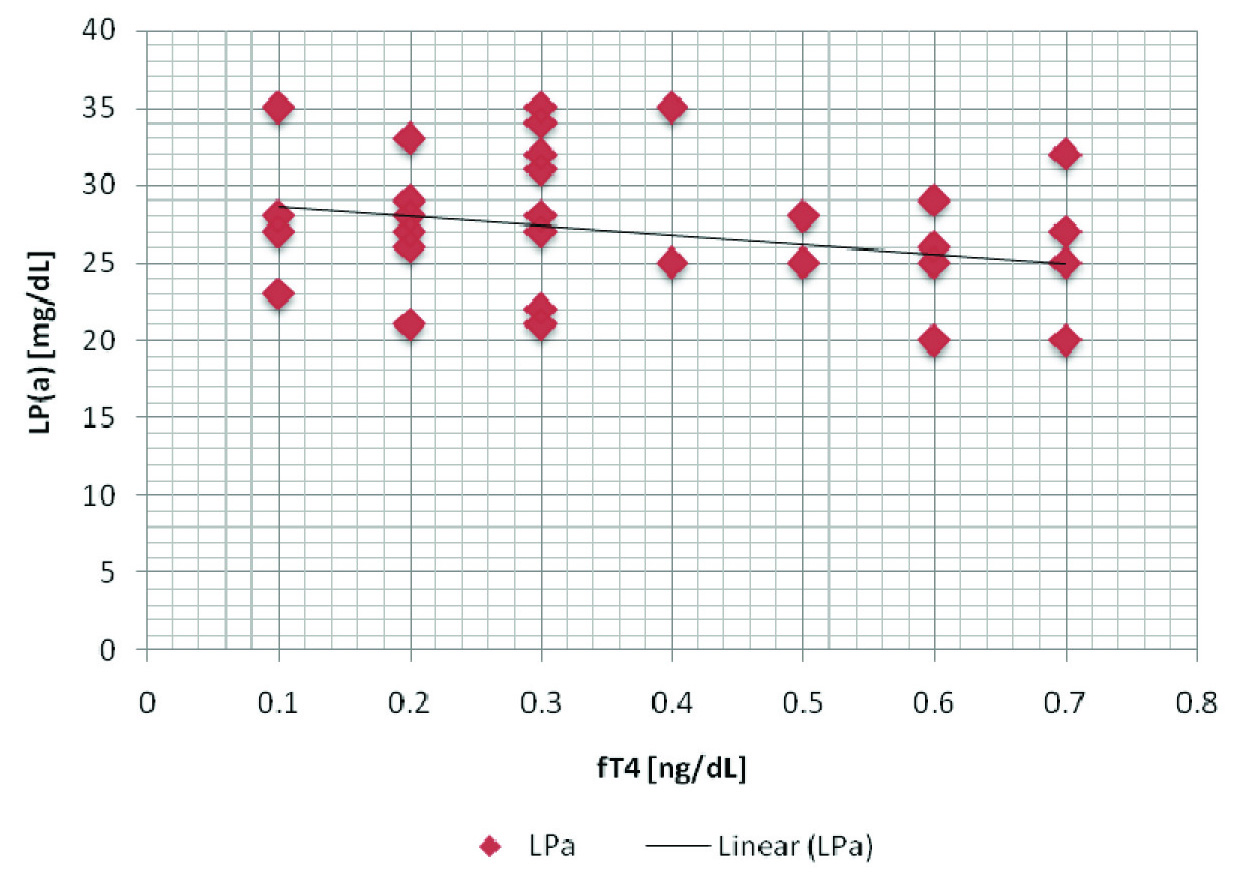
Though levels of FT4 and HDL - C were lower as compared to those of controls, we found higher levels of LDL - C, LDL/HDL - C, Lp(a), non-HDL, TC, TG, TSH and VLDL in patient group [Table/Fig-2], with a high statistical significance. LP(a) showed a positive correlation with TSH, LDL and non-HDL [Table/Fig-3], as has been depicted through the correlation graphs [Table/Fig-4,5]. Whereas a negative correlation was observed between LP(a) and FT4 levels in hypothyroid patients [Table/Fig-6]. These results thus provided valuable information that LP(a) had a strong relationship with thyroid hormones and alterations in it could reflect the levels of thyroid hormone in thyroid patients.
Correlation between parameters in hypothyroid patients
| Parameters | r-Value | p-Value |
|---|
| FT4 & Lp(a) | -0.303 | 0.03* |
| Lp(a) & TSH | 0.320 | 0.02* |
| Lp(a) & LDL | 0.282 | 0.04* |
| Lp(a) & non-HDL | 0.305 | 0.03* |
| LDL & non-HDL | 0.880 | 0.0001** |
* Correlation is significant at the 0.05 level (2-tailed)
NS: Not significant,**Correlation is significant at the 0.01 level (2-tailed)
A positive correlation between TSH and Lp(a) in hypothyroid patients Lp(a) - Upto 14 mg/dL; TSH - 0.5 - 5.0 μIU/mL
A positive correlation between LP(a) and non-HDL in hypothyroid patients Lp(a) - Upto 14 mg/dL; non- HDL - < 130 mg/dL
A negative correlation between fT4 and Lp(a) in hypothyroid patients fT4 - reference range, 0.8 - 1.8 ng/dL; Lp(a) - Upto 14 mg/dL
Discussion
Lipoprotein(a) [Lp(a)] is a cholesterol-enriched lipoprotein particle, which is receiving growing attention as a potential, independent risk factor for cardiovascular diseases [15]. Several studies, including the present one, have indicated an interaction between Lp(a) and LDL levels [16–24]. Our study showed a significant positive correlation between Lp(a) and LDL in hypothyroid patients, suggesting that various mechanisms such as a direct interaction of apo(a) with other apoB100-containing particles facilitated lipoprotein aggregate formation [25], or the trapping of LDL particles after Lp(a) bound to proteoglycans of the arterial wall by its apoB component, or after uptake of Lp(a) particle by macrophages after contact of these cells with minimally oxidized LDL [26]. These could play a role in contributing to the rise in Lp(a) levels.
There is a preponderant genetic influence that supports the concept of metabolic independence and hypothyroidism could have little effect on Lp(a) levels. However, Lee et al., observed no significant differences in serum Lp(a) levels in different thyroid function groups [27]. Although there are contradictory data on the fluctuations in Lp(a) levels, some authors [18,19] think that they are affected by thyroid function, and several others think that they are unaffected [17, 20, 26]. Our study done on hypothyroid patients showed a negative correlation between fT4 and Lp(a), which was contradictory to the findings of study conducted by Lee et al.
The potential association between Lp(a) and thyroid function status in the general population might be regarded as an important aspect for cardiovascular risk prediction and prevention. We and some other authors have pointed out the correlation between TSH (47.82±4.39) and Lp(a) (27.02±0.58) levels in non-treated hypothyroid subjects, which suggested the influence of thyroid hormones on Lp(a) metabolism. This hypothesis was supported by De Bruin et al., [7], who demonstrated an almost perfect correlation between free thyroxine index and Lp(a).
The correlation analysis in our study also revealed that there was an influence of TSH on Lp(a) levels in hypothyroid patients. Our findings, in combination with previously reported data, therefore, suggest that in severe hypothyroid state, an impairment of the catabolism of the apo B-containing particles, including Lp(a), predominates; and that an elevated secretion of apo B and Lp(a), probably in combination with decreased catabolism, might be postulated. This influence could have been caused by lesser lipolytic activity of lipase enzymes [28].
Studies done on non–HDL-C by Edward J. Shahady showed that it was a more precise therapeutic target than LDL-C [29]. In our study, the positive correlation between LDL and non-HDL-C and with Lp(a) revealed that non - HDL-C could serve as an excellent proxy for assessing the total number of circulating atherogenic particles. Our study may also suggest that non–HDL-C may be superior to LDL-C in predicting cardiovascular disease and that it can be used as the primary lipid target, with patients having characteristic dyslipidaemia that consisted of decreased HDL-C levels, elevated TG levels, and normal to elevated LDL-C levels.
Conclusion
Hypothyroidism severely disturbs the lipid homeostasis in liver and adipose tissue, thus contributing to an alteration in circulating lipids, as was expressed in our correlation study. Our present findings indicated that hypothyroidism could be strongly associated with lipid abnormalities that enhanced the development of cardiovascular diseases.
Though our study provided clear insights on lipid parameters and their levels, in future, studies done should be focused on understanding the molecular mechanisms behind the dependence or independence on thyroid receptors for regulation of target genes which are involved in lipid homeostasis, that may entail therapeutic potentials, not only for the prevention and treatment of thyroid disorders, but also for prevalent diseases in the world, such as obesity and cardiovascular disease.
As an outcome of this correlation study, a recommended screening can be advised for any patient with thyroid dysfunction, especially hypothyroidism, to assess lipid abnormalities by using Lp(a) and non- HDL – C, which may prevent them from secondary diseases. Vice versa, in any case of unexplained hyperlipdaemia, thyroid screening can be done to assess the underlying disease.
*Correlation is significant at the 0.05 level (2-tailed).
NS: Not significant,**Correlation is significant at the 0.01 level (2-tailed)
*Correlation is significant at the 0.05 level (2-tailed)
NS: Not significant,**Correlation is significant at the 0.01 level (2-tailed)
* Correlation is significant at the 0.05 level (2-tailed)
NS: Not significant,**Correlation is significant at the 0.01 level (2-tailed)
[1]. Utermann G, Lipoprotein (a). In: Scriber CR, Beaudet AL, Aly WS, Vallo O, editorsThe Metabolic and Molecular Bases of Inherited Disease 2001 8th edNew York, NYMcGraw-Hill:2753-87. [Google Scholar]
[2]. Murase T, Okubo M, Amemiya-kudo M, Impact of markedly elevated serum lipoprotein(a) levels (>or = 100 mg/dl) on the risk of coronary heart diseaseMetabolism 2007 56:1187-91. [Google Scholar]
[3]. Erqou S, Kaptoge S, Perry PL, Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortalityJAMA 2009 302:412-23. [Google Scholar]
[4]. Nordesgaard BG, Chapman MJ, Ray K, Lipoprotein(a) as a cardiovascular risk factor: current statusEur Heart J 2010 31:2844-53. [Google Scholar]
[5]. Brown WV, Christie M, Ballantyne CM, Jones PH, Marcovina S, Management of Lp(a)J Clin Lipidol 2010 4:240-47. [Google Scholar]
[6]. Engler H, Riesen WF, Effect of thyroid function on concentrations of lipoprotein(a)Clin Chem 1993 39:2466-69. [Google Scholar]
[7]. De Bruin TW, van Barlingen van Linde-Sibenius Trip M, Lipoprotein(a) and apolipoprotein B plasma concentrations in hypothyroid, euthyroid, and hyperthyroid subjectsJ Clin Endocrinol Metab 1993 76:121-26. [Google Scholar]
[8]. Becerra A, Bellido D, Luengo A, Piédrola G, De Luis DA, Lipoprotein(a) and other lipoproteins in hypothyroid patients before and after thyroid replacement therapyClin Nutr 1999 18:319-22. [Google Scholar]
[9]. Tzotzas T, Krassas GE, Konstantinidis T, Bougoulia M, Changes in lipoprotein(a) levels in overt and subclinical hypothyroidism before and during treatmentThyroid 2000 10:803-08. [Google Scholar]
[10]. Spandrio S, Sleiman I, Scalvini T, Salvi A, Di Stefano O, Pagliaini R, Balestrieri GP, Lipoprotein(a) in thyroid dysfunction before and after treatmentHorm Metab Res 1993 25:586-89. [Google Scholar]
[11]. Pazos F, Alvarez JJ, Rubies-Prat J, Varela C, Lasuncion MA, Long term thyroid replacement therapy and levels of lipoprotein (a) and other lipoproteinsJ Clin Endocrinol Metab 1995 80:562-66. [Google Scholar]
[12]. Arem R, Escalante DA, Arem N, Morrisett JD, Patsch W, Effect of L-thyroxine therapy on lipoprotein fractions in overt and subclinical hypothyroidism, with special difference to lipoprotein(a)Metabolism 1999 44:1559-63. [Google Scholar]
[13]. Ito M, Arishima T, Kudo T, Effect of levo-thyroxine replacement on non-high-density lipoprotein cholesterol in hypo thyroid patientsJ Clin Endocrinol Metab 2007 92:608-11. [Google Scholar]
[14]. Lippi G, Guidi G, Lipoprotein(a): an emerging cardiovascular risk factorCrit Rev Clin Lab Sci 2003 40:1e42 [Google Scholar]
[15]. Gerald Luc, Lipoprotein (a) as a predictor of coronary heart disease: the PRIME StudyAtherosclerosis 2002 163:377-84. [Google Scholar]
[16]. Cantin B, Gagnon F, Moorjani S, Despres JP, Lamarche B, Lupien PJ, Is lipoprotein(a) an independent risk factor for ischemic heart disease in men? The Quebec cardiovascular studyJ Am Coll Cardiol 1998 31:519-25. [Google Scholar]
[17]. Dahlen GH, Weinehall L, Stenlund H, Jansson JH, Hallmans G, Huhtasaari F, Lipoprotein(a) and cholesterol levels act synergistically and apolipoprotein A-I is protective for the incidence of primary acute myocardial infarction in middle-aged males. An incident case-control study from SwedenJ Intern Med 1998 244:425-30. [Google Scholar]
[18]. Armstrong VW, Cremer P, Eberle E, The association between serum Lp(a) concentrations and angiographically assessed coronary atherosclerosis. Dependence on serum LDL levelsAtherosclerosis 1986 62:249-57. [Google Scholar]
[19]. Maher VMG, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ, Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a)JAMA 1995 274:1771-4. [Google Scholar]
[20]. von Eckardstein A, Schulte H, Cullen P, Assmann G, Lipoprotein(a) further increases the risk of coronary events in men with high global cardiovascular riskJ Am Coll Cardiol 2001 37:434-9. [Google Scholar]
[21]. Schaefer EJ, Lamon Fava S, Jenner JL, Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid Research Clinics Coronary Primary Prevention TrialJAMA 1994 271:999-1003. [Google Scholar]
[22]. Cremer P, Nagel D, Labrot B, Mann H, Muche R, Elster H, Lipoprotein Lp(a) as predictor of myocardial infarction in comparison to fibrinogen, LDL cholesterol and other risk factors: results from the prospective Gottingen Risk Incidence and Prevalence Study (GRIPS)Eur J Clin Invest 1994 24:444-53. [Google Scholar]
[23]. Berg K, Dahlen G, Christophersen B, Cook T, Kjekshus J, Pedersen T, Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival StudyClin Genet 1997 52:254-61. [Google Scholar]
[24]. Seed M, Hoppichler F, Reaveley D, McCarthy S, Thompson GR, Boerwinkle E, Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemiaN Engl J Med 1990 322:1494-9. [Google Scholar]
[25]. Haberland ME, Fless GM, Scanu AM, Fogelman AM, Malondialdehyde modification of lipoprotein(a) produces avid uptake by human monocyte-macrophagesJ Biol Chem 1992 267:4143-51. [Google Scholar]
[26]. Alfthan G, Pekkanen J, Jauhiainen M, Relation of serum homocysteine and lipoprotein(a) concentrations to atherosclerotic disease in a prospective Finnish population based studyAtherosclerosis 1994 106:9-19. [Google Scholar]
[27]. Lee WY, Suh JY, Rhee EJ, Park JS, Sung KC, Kim SW, Plasma CRP, apolipoprotein A-1, apolipoprotein B and Lpa levels according to thyroid function statusArch Med Res 2004 35:540e545 [Google Scholar]
[28]. Packard CJ, Shepherd Lindsay GM, Gaw A, Taskinen MR, Thyroid replacement therapy and its influence on postheparin plasma lipases and apolipoprotein-B metabolism in hypothyroidismJ Clin Endocrinol Metab 1993 76:1209-1216. [Google Scholar]
[29]. Shahady Edward J, Non-HDL Cholesterol: When—and How—to TreatConsultant September 2008 [Google Scholar]