Effect of Smoking on Metalloproteinases (MMPs) Activity in Patients with Acute Myocardial Infarction (AMI)
Sreekanth K Sivaraman1, Geevar Zachariah2, PT Annamala3
1 Professor, Department of Biochemistry, Sree Gokulam Medical College & Research Foundation, Venjaramoodu P.O., Thiruvananthapuram, Kerala, India.
2 Consultant Cardiologist, Mother Hospital, Pullazhi P.O., Olari, Thrissur, Kerala, India.
3 Professor, Department of Biochemistry, Jubilee Mission Medical College & Research Institute, P.B. No: 737, Thrissur, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sreekanth K. Sivaraman, Professor, Department of Biochemistry, Sree Gokulam Medical College & Research Foundation, Venjaramoodu P.O., Thiruvananthapuram-695 607, Kerala, India.
Phone: 09895058153,
E-mail: sree9in@rediffmail.com
Introduction: Many risk factors are involved in the course and pathogenesis of Acute Myocardial Infarction (AMI). Smoking can significantly increase the AMI mortality and morbidity. Matrix metalloproteinases (MMPs), a class of Zn containing enzymes, are involved in the erosion of the fibrous cap and rupture of the plaque which leads to AMI.
Aim: To evaluate the activity of MMP2 and MMP9 in AMI patients, with or without the habit of smoking.
Materials and Methods: The study group consists of 300 AMI patients and 100 sex and age matched control subjects with and without the habit of smoking. MMP2 and MMP9 activities were measured in the blood samples of these patients and controls by sandwich enzyme immunoassay and the values were noted and compared.
Results: Both MMP2 and MMP9 were found to be significantly elevated in all the AMI patients when compared to the normal controls subjects irrespective of the habit of smoking. However MMP9 showed a significant elevation when compared to MMP2 in patients with the habit of smoking.
Conclusion: The results of the present study shows increased concentration of both MMPs in AMI patients. However, concentration of MMP9 was found to be more in patients with the habit of smoking when compared to MMP2, indicating that smoking can increase the activity of MMP9 in these patients. Hence apart from producing the free radicals, the smoke can increase the activities of matrix degrading enzymes which in turn contribute to the vulnerability of plaque.
Acute Myocardial Infarction, Matrix Metalloproteinases
Introduction
Recent studies have suggested that the different phases of atherosclerosis and its sequlae AMI may be mediated by the family of MMPs [1], Zn dependent physiological regulators of the extra cellular matrix. These Zn containing endopeptidases have functions in the normal and injury-induced turnover of the extracellular matrix [2]. Though it is not completely certain how MMP production is induced by the extra cellular matrix, these gelatinases are necessary for infiltration of monocytes and T-lymphocytes to occur in the sub endothelial spaces [3]. Activated endothelial cells express adhesion molecules, such as vascular cell adhesion molecule-1, which promote infiltration of circulating monocytes and T-lymphocytes and adhesion of these cells to the endothelial cells could induce production of MMP2 (72 KD, Gelatinase A), which facilitates breakdown of the extra cellular matrix [4]. More over contact with type I collagen and laminin in the matrix increases expression of MMP9 (92 KD, Gelatinase B), and inter leukin-1 (IL-1) secretion can activate pro-MMP2 and pro-MMP9 produced by smooth muscle cells, which could lead to endothelial disruption or intra plaque hemorrhage [3].
Of the several risk factors of AMI, smoking can significantly increase coronary artery disease (CAD) mortality and morbidity, an adverse effect related to the amount of tobacco smoked daily and the duration of smoking [5]. Passive smoking has also been found to increase CAD risk and the impact of smoking on CAD risk is modified by plasma lipid levels [6]. Nicotine stimulates release of adrenaline leading to increased serum concentrations of fatty acids [7]. Free fatty acid is a stimulant of hepatic secretion of LDL and TGs. The free fatty acid can also stimulate hepatic synthesis and release of cholesterol [8]. In addition to this cigarette smoking can alter coagulation system, produce various free radicals [9]; all of which may contribute to atherosclerosis. The benefit of smoking cessation is seen regardless of how long and how much the person previously smoked [10].
Being a small State in the southern part of India, the incidence of AMI has tremendously increased in Kerala for the past few years. The causes for this may be the lifestyle and the increased rate of smoking irrespective of the age. Hence the present study was undertaken to evaluate the activity of MMP2 and MMP9 in AMI patients, with or without the habit of smoking.
Materials and Methods
A total of 300 AMI patients and 100 age and sex matched control subjects were enrolled in this case control study. From these patients and controls, the study groups were selected based on their habit of smoking and the present study included both smokers and non smokers with AMI and their respective controls. The study included 222 AMI patients with the habit of smoking along with 62 normal controls with the habit of smoking but without the presentation of AMI. Non-smokers (n=78) with AMI were also studied along with 38 normal controls without the habit of smoking and without the presentation of AMI.
All patients admitted to the Intensive Coronary Care Unit (ICCU) with a diagnosis of AMI presenting within 24 hours of onset of chest pain were included in the study and myocardial infarction was diagnosed in these patients by at least 0.1-mv ST segment elevation in two or more contiguous limb leads or 0.2-mv ST segment elevation in two or more chest leads associated with typical chest pain. We have included only the patients diagnosed with AMI. Exclusion criteria were patients with cardiogenic shock, cerebrovascular accident, significant hepatic or renal diseases, clear evidence of infection anywhere in the body. The normal volunteers had no past history or evidence of cardiovascular disease, hypertension or diabetes mellitus. The present study does not include control subjects with a history of neoplastic, hepatic, infectious or autoimmune disease or any surgical procedure in the preceding 6 months. Written informed consent was obtained from each subjects prior to the study. The study was started only after obtaining the ethical clearance from the institutional ethics committee. The work was carried out in the Department of Cardiology at Amala Cancer Hospital and Research Centre, Thrissur, Kerala, India.
In patients included in the study, detailed history was taken and complete physical examination was carried out. A 12 lead ECG with V3R and V4R was recorded immediately on admission and repeated after 2hrs, 6hrs, 12hrs, 24hrs, 48hrs and pre-discharge. 10 ml blood was withdrawn for laboratory analysis from the peripheral vein on admission in heparinised tubes. After centrifugation the plasma samples were frozen and stored at -80oC until the MMP assay was carried out. Serial creatine kinase assays were also done to confirm the myocardial infarction and it was done at 2hrs, 6hrs, 12hrs, 24hrs, and 48hrs after admission. Chest X- ray was done at the time of discharge from the ICCU. An echocardiographic examination was performed at the time of discharge or at the end of the first week or early second week after admission.
The patients were allowed to relax and on the second day after the admission they were subjected to an oral questionnaire as described in our Performa, in order to collect the history of these patients and also to find out whether they have the habit of smoking.
The blood collected from the patients at the time of admission was used for the assay of MMPs. MMP assay is based on the principle that the antibodies immobilized on a bead matrix, in combination with enzyme-labeled antibodies, directed against different antigenic sites on the same MMP molecule [11,12]. Upon addition of an MMP containing specimen, the result is an MMP molecule being sandwiched between the solid phase and enzyme labeled antibodies. After removing unbound enzyme-labeled antibody, the bead containing the sandwich is incubated with enzyme substrate and O-phenylenediamine, resulting in the development of color. The activity of the peroxidase enzyme is proportional to the amount of antigen, MMP, so that MMP concentration in specimens can be determined from a standard curve.
The assay mixture contained 50μl of standard or specimen with 300μl enzyme labeled antibody solution and one anti-MMP coated bead. The mixture was incubated at 17-270C for 1 hour. The reaction was then stopped by the addition of 3ml of washing solution and it was aspirated and this was repeated at least 3 times. Each washed bead was then transferred in to a clean fresh tube and 300μl of coloring solution was added and incubated at 17-270C for 1 hour. The reaction was then stopped by the addition of 1.5ml of stop solution. Using deionized water as blank, the absorbance for the standard curve solutions and specimens were taken at 492 nm (A492).
Statistical Analysis
The statistical analysis was done by using Sigma stat version 2 and the values obtained were expressed as mean + SD . Statistical significance was done by using the z-test and by the analysis of variance (ANOVA). The z-value was determined and the results obtained by z-test were expressed in terms of probability (p). z-value > 2.88 (p<0.01) and >1.96 (p<0.05) were considered to be statistically significant. The inter group comparison have been done by using the one-way analysis of variance (ANOVA) using the same programme.
Results
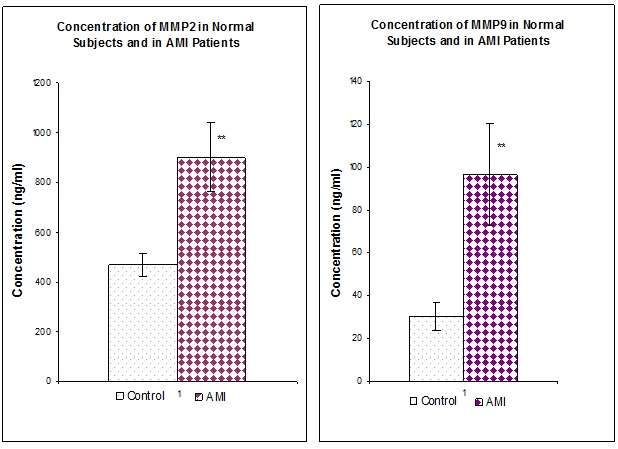
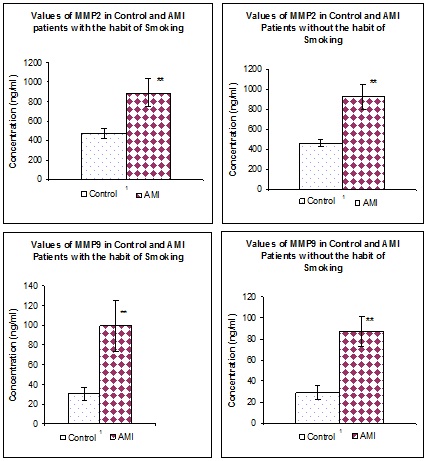
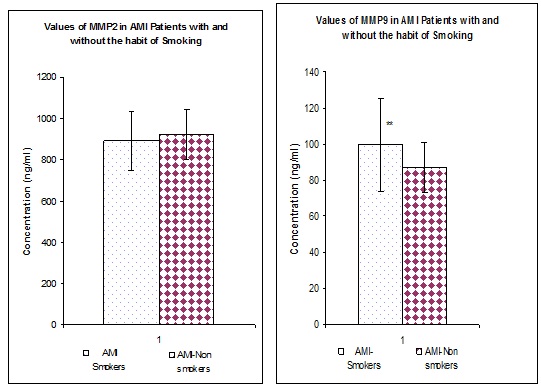
[Table/Fig-1] represents the values of MMP2 and MMP9 in both normal as well as in AMI patients irrespective of the smoking habits. When compared to the normal individuals (n=100) the values of both MMPs were found to be significantly (p<0.01) increased in all the AMI patients (n=300). [Table/Fig-2] represents the values of MMP2 and MMP9 in both normal as well as in AMI patients with and without the habit of smoking. Irrespective of the smoking habit, Both MMPs were found to be significantly elevated (p<0.01) in AMI patients when compared to their respective controls. [Table/Fig-3] represents the Values of MMP2 and MMP9 in AMI patients with and without the habit of smoking. MMP2 does not show any significant difference in patients with and without the habit of smoking. Whereas MMP9 was significantly (p<0.01) elevated in AMI patients with the habit of smoking when compared to AMI patients without the habit of smoking.
Values of MMP2 and 9 in normal subjects and in AMI patients, values are expressed as Mean + SD, **p<0.01
Values of MMP2 and MMP9 in control and in AMI patients with and without the habit of smoking, Values are expressed as Mean + SD, **p<0.01
Values of MMP2 and MMP9 in AMI patients with and without the habit of smoking, values are expressed as mean + SD, **p<0.01
Discussion
Cigarette smoking independently increases the coronary atherosclerotic disease, cerebrovascular disease and peripheral vascular diseases [13]. Inflammation and arterial wall oxidative stress are central in the pathogenesis of atherosclerosis [14]. The ability of cigarette smoke to induce vascular inflammation and oxidative stress appears fundamental to the broad effects of smoking on vascular pathophysiology [15]. The well described role of MMPs in the pathogenesis of smoking related chronic obstructive pulmonary disease serves as a paradigm for considering the potential role of MMPs in smoking related Cardio Vascular Diseases [16]. Activated macrophages, mast cells, T-lymphocytes, endothelial cells and vascular smooth muscle cells are the principle sources of MMPs in the vasculature. Cigarette smoke by increasing vascular inflammation and vascular ROS has the potential to increase the MMP expression by each of these cell types [17]. This is well evident from the present study as both MMP2 and MMP9 are found to be significantly elevated in all the AMI patients when compared to normal healthy individuals. This could indicate increased proteolysis in the plaques by an enhanced expression of these MMPs. The studies conducted by Kai H et al., also indicate increased expression of MMP2 and MMP9 in patients with acute coronary syndrome[18], supporting our findings. MMP system components are expressed in atherosclerotic tissues and in their active form they may contribute to vascular remodeling and plaque disruption [19].
However there are certain important findings obtained from this study. Both MMP2 and MMP9 were found to be elevated in all the AMI patients with and without the habit of smoking and out of these two MMPs, MMP9 was found to be predominantly elevated in AMI patients with the habit of smoking than MMP2 in patients with the habit of smoking. These findings are similar to the findings obtained by the study conducted by Garvin P et al., from Sweden [20]. They have demonstrated the elevated levels of circulating MMP-9 in middle aged normal population with cardiovascular risk factors including cigarette smoking. The significant elevation of MMP9 in AMI smokers when compared to that of MMP2 may be due to the increased expression of MMP9 in vasculature and also due to the availability of its substrate (type IV collagen) in the atherosclerotic plaque, which can contribute to the increased activity of MMP9. Further cigarette smoke could induce and inhibit the MMPs activity at multiple levels [21]. Presence of reactive oxygen species (ROS) and decreased nitric oxide activity due to cigarette smoke can induce the MMP transcription [22,23] and which may be another reason for the increased expression of this MMP. The monocyte interaction with collagen and platelets is required for the leukocytes to synthesize MMP9 and that the interactions are produced particularly in areas of the vessel where inflammatory phenomena develop in response to injury [24], especially by smoking. Thus MMP9 plays a significant role in the progress of extra cellular matrix degradation in the plaques [25] in AMI patients with the habit of smoking.
Epidemiological studies have shown that MMP9 levels are elevated in the circulation of patients with acute coronary syndromes [26]. Studies have also shown the increased expression of MMP9 along with other MMPs in endothelial cells exposed to cigarette smoke condensate [27]. Activity of MMP is regulated at the levels of gene transcription, proenzyme activation and endogenous inhibitors of MMPs. Cigarette smoke induced inflammation and oxidative stress have the potential to induce and inhibit MMP activity at the multiple levels. Cigarette smoke may increase MMP expression via activation of inflammatory transcription factors [28]. It is also associated with increased circulating levels of TNF-alpha and IL-1beta as well as increased monocyte expression of IL-1beta [29]. Nicotine and cotinine of the cigarette smoke directly stimulates vascular smooth muscle cells (VSMCS) collagenase, stromelysin and gelatinase expression, which will lead to plaque rupture [30]. This is well-evident from our studies as our results show increased activity of both MMP2 and MMP9 in all the AMI patients and also the increased activity of MMP9 in AMI patients with the habit of smoking.
Conclusion
Through the present study we could able to prove the increased activity of both MMP2 and MMP9 in AMI patients. Further the activity of MMP9 was found to be more in AMI patients with the habit of smoking and can be used as a risk predictor for future AMI as well as a potential indicator of AMI in subjects with the habit of smoking.
[1]. Brown DL, Hibbs MS, Kearny M, Loushin C, Isner JM, Identification of 92 KD gelatinase in human coronary atherosclerotic lesionsCirculation 1995 91(2125-31) [Google Scholar]
[2]. Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G, Biochemistry and molecular biology of gelatinase B or matrixmetalloproteinase-9 (MMP-9)Crit Rev Biochem Mol Biol 2002 37:375-536. [Google Scholar]
[3]. Beatriz Alvarez, Carmen Ruiz, Pilar Chacon, Jose Alvarez-Sabin, Manuel Matas, Serum values of metalloproteinase 2 and metalloproteinase 9 as related to unstable plaque and inflammatory cells in patients with greater than 70% carotid artery stenosisJ Vas Surg 2004 40(3):469-75. [Google Scholar]
[4]. O’Brien KD, Allen MD, Mc Donald TO, Chait A, Harlan JM, Fishbein D, Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques implications for the mode of advanced coronary atherosclerosisJ Clin Invest 1993 92:945-51. [Google Scholar]
[5]. Wilhelmsen L, Coronary Heart Disease: Epidemiology of smoking and intervention studies of smokingAm Heart J 1998 115:242-49. [Google Scholar]
[6]. Yano K, Reed DM, McGee DL, Ten year incidence of coronary heart disease in the Honolulu Heart Programme: relationship to biological and life style characteristicsAm J Epidemiol 1984 119:653-66. [Google Scholar]
[7]. Shepherd J, Packard CJ, Patch JR, Goth AM, Tanmon OD, Effect of dietary polyunsaturated and saturated fat on the properties of high-density lipoproteins and the metabolism of apolipoproteinA J Clin Invest 1978 61:1582-92. [Google Scholar]
[8]. Banonome A, Pagnon A, Biffanti S, Opportuno A, Sorgato F, Dorella M, Maiorono M, Ursini F, Effect of dietary mono unsaturated and poly unsaturated fatty acids on the susceptibility of plasma low density lipoproteins to oxidative modificationsArterioscler Thromb 1992 12:529-33. [Google Scholar]
[9]. Sreekanth Kavitha Sivaraman, Sabu Mandumpal Chacko, Varghese Leyon, Manesh Chittezhath, Kuttan Girija, Kuttan Ramadasan, Antioxidant activity of smoke shield in-vitro and in-vivoJ Pharm Pharmacol 2003 55:847-53. [Google Scholar]
[10]. Kawachi J, Kaplan GA, Goldberg DE, Salonen JT, Beer drinking and mortality results from the kuopio ischaemic heart disease risk factor study, a prospective population based studyBrit Med J 1997 315:846-51. [Google Scholar]
[11]. Fujimoto N, Mouri N, Iwata K, Ohuchi E, Hayakawa T, A one step sandwich enzyme immunoassay for human matrix metalloproteinase 2 (72-kDa gelatinase / type IV collagenase) using monoclonal antibodiesClinica Chimica Acta 1993 231:91-103. [Google Scholar]
[12]. Fujimoto N, Hosokawa N, Iwata K, Shinya T, Okada Y, Hayakawa T, A one step sandwich enzyme immunoassay for inactive precursor and complexed forms of human matrix metalloproteinase 9 (92-kDa gelatinase / type IV collagenase, gelatinase B) using monoclonal antibodiesClinica Chimica Acta 1994 231:79-88. [Google Scholar]
[13]. O’Donnell CJ, Kannel WB, Epidemiology of Atherosclerotic Vascular Disease. In: Lanzer PT, Toppel EJ edsPanvascular Medicine 2003 BerlinSpringer –Verlag [Google Scholar]
[14]. Ross R, Atherosclerosis is an inflammatory diseaseAm Heart J 1999 138:S419-S420. [Google Scholar]
[15]. Ambrose JA, Barua RS, The pathophysiology of cigarette smoking and cardio vascular disease: an updateJ Am Coll Cardiol 2004 43:1731-37. [Google Scholar]
[16]. Shapiro SD, Proteolysis in the lungEur Respir J Suppl 2003 44:S30-S32. [Google Scholar]
[17]. Chase AJ, Newby AC, Regulation of matrix metalloproteinase (matrixin) genes in blood vessels: a multi-step recruitment model for pathological remodelingJ Vas Res 2003 40:329-343. [Google Scholar]
[18]. Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Peripheral blood levels of matrix metalloproteinase-2 and -9 are elevated in patients with acute coronary syndromesJ Am Coll Cardiol 1998 32:368-72. [Google Scholar]
[19]. Galis ZS, Sukhova GK, Kranzhofer R, Clark S, Libby P, Macrophage foam cells from experimental atheroma constitutively produce matrix degrading proteinasesProc Natl Acad Sci USA 1995 92:402-06. [Google Scholar]
[20]. Garvin P, Nilsson L, Carstensen J, Jonasson L, Kristenson M, Circulating matrix metalloproteinases-9 is associated with cardiovascular risk factors in a middle-aged normal populationPloS ONE 2008 3:1774 [Google Scholar]
[21]. Wright JL, Levy RD, Churg A, Pulmonary hypertension in chronic obstructive pulmonary disease: current theories of pathogenesis and their implications for treatmentThorax 2005 60:605-09. [Google Scholar]
[22]. Galis ZS, Asanuma K, Godin D, Meng X, N-acetyl cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells: new target for antioxidant therapy?Circulation 1998 97:2445-53. [Google Scholar]
[23]. Chen HH, Wang DL, Nitric oxide inhibits matrix metalloproteinase-2 expression via the induction of activating transcription factor 3 in endothelial cellsMol Pharmacol 2004 65:1130-40. [Google Scholar]
[24]. Galt SW, Lindemann S, Medd D, Allen LL, Kraiss LW, Harris FS, Differential regulation of matrix metalloproteinases 9 by monocytes adherent to collagen and plateletsCirc Res 2001 89:509-16. [Google Scholar]
[25]. Lutgens E, Cleutjens KB, Heeneman S, Both early and delayed anti- CD 40 L antibody treatment induce a stable plaque phenotypeProc Natl Acad Sci USA 2000 97:7494-99. [Google Scholar]
[26]. Inokubo Y, Hanada H, Ishizaka H, Fukushi T, Kamada T, Okumura K, Plasma levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 are increased in the the coronary circulationin patients with acute coronary syndromeAm Heart J 2001 141:211-17. [Google Scholar]
[27]. Nordskog BK, Blixt AD, Morgan WT, Fields WR, Hellmann GM, Matrix-degrading and pro-inflammatory changes in human vascular endothelial cells exposed to cigarette smoke condensateCardiovasc Toxicol 2003 3:101-17. [Google Scholar]
[28]. Perlstein Todd S, Lee Richard T, Smoking, Metalloproteinases and Vascular DiseaseArterioscler Thromb Vasc Biol 2006 26:250-56. [Google Scholar]
[29]. Ryder MI, Saghizadeh M, Ding Y, Nguyen N, Soskolne A, Effects of tobacco smoke on the secretion of interleukin-1 beta, tumor necrosis factor-alpha and transforming growth factor-beta from peripheral blood mononuclear cellsOral Microbiol Immunol 2002 17:331-36. [Google Scholar]
[30]. Carty CS, Soloway PD, Kayastha S, Bauer J, Marasan B, Ricotta JJ, Dryjski M, Nicotine and cotinine stimulate secretion of basic fibroblast growth factor and affect expression of matrix metalloproteinases in cultured human smooth muscle cellsJ Vas Surg 1996 24:927-34. [Google Scholar]