Can Treatment for Polycystic Ovarian Disease Induce Ovarian Tumour? A Case Report
Gowri Dorairajan1, N. Hima Bindu2, Ramachandra V Bhat3
1Professor, Department of Obstetrics and Gynecology,JIPMER, Puducherry, India.
2Associate Professor. Department of Obstetrics and Gynecology,Indira Gandhi Medical College, RI, Puducherry, India.
3Professor, Department of Pathology,Indira Gandhi Medical College, RI, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Gowri Dorairajan, No. 68,1st Cross Street, Nanbargal Nagar Reddiarpalayam, Puducherry 605010, India.
Phone: +919894348518
E-mail: gowridorai@hotmail.com
Polycystic ovarian disease in adolescents is not uncommon. Usually underlying ovarian tumours can cause hirsutism and ovulation. A 15-year-old girl presented with infrequent cycles and hirsutism. Her baseline evaluation ruled out ovarian tumour and other endocrinological problems. She was treated with insulin sensitizers and hormone treatment. After more than a year of treatment she developed a large ovarian tumour which turned out to be a juvenile granulose cell tumour at laparotomy a year after treatment with insulin sensitizers. The authors recommend continued surveillance of ovaries of adolescent girls undergoing treatment for polycystic ovarian disease to monitor for formation of ovarian tumours.
Polycystic ovarian disease, Juvenile granulose cell tumour, Hirsutism, Anovulation
Case Report
A 15-year-old girl presented with severe hirsutism and oligomenorhoea. She had attained menarche 3 years back and her cycles were irregular. The menstrual cycle was once in 3-4 months with the flow manifesting mostly after progesterone withdrawal and lasting 5 days. There was no history suggestive of hypothyroidism, excess weight gain, any chronic illness or surgeries in the child hood. She was born to non consanguineous couple by normal delivery with an average birth weight. Her younger sibling was 8 years and had not attained menarche.
On examination she had a Ferriman-Gallwey Scoring of 11 for hirsutism with the upper lip, chin and the lower abdomen maximally affected. Her Body mass index (BMI) was 20. Her breast development was Tanner grade IV. There was no nipple discharge. There was no Acanthosis Nigricans. The abdomen and the external genitalia were normal. There was no clitoromegaly.
Investigations
Plasma free fasting insulin level was 11.74 μunit/ml (within normal limits). Total testosterone (TT), and rostendione (A), dehydroepiandrosterone sulphate (DHEAS) and 17-hydroxyprogesterone (17-OHP) were within normal limits.Thyroid function was normal. Free plasma TT was 46.2 ng/dl. Serum prolactin was 11.12ng/ml. The glucose tolerance test was normal. Ultrasound ruled out ovarian tumour and the morphology was characteristic of polycystic ovaries.
She was started on insulin sensitizer Metformin tablets 1000mg a day, in two divided doses and gradually increased to 1500mg, as she tolerated it well. She was started on combined oral estrogen progesterone combination (30 Micro Gram ethinyl estradiol and 0.15mg levonorgestrel) for 6 months. The menstrual cycles spontaneously regularized for another 3 cycles with insulin sensitizers alone. Thereafter, the patient was advised self administration of progesterone withdrawal every 8 weeks. Hirsutism was treated with epilation followed by laser treatment by a dermatologist. She presented back to us 18 months later in late February 2012 with heaviness in the lower abdomen, and frequent micturition. Her last withdrawal bleed was 4 weeks prior.
Abdominal examination revealed a large mobile non-tender cystic mass arising from pelvis measuring 30×25 cm. There was no free fluid. Sonography confirmed a multiloculated cystic mass arising from the right ovary. There were no solid areas or free fluid and the left ovary appeared normal. The uterus was normal and the endometrial thickness was 10 mm.
Repeat investigations revealed highly elevated free TT (460ng/dl), marginally elevated alpha feto protein and lactic dehydrogenase.
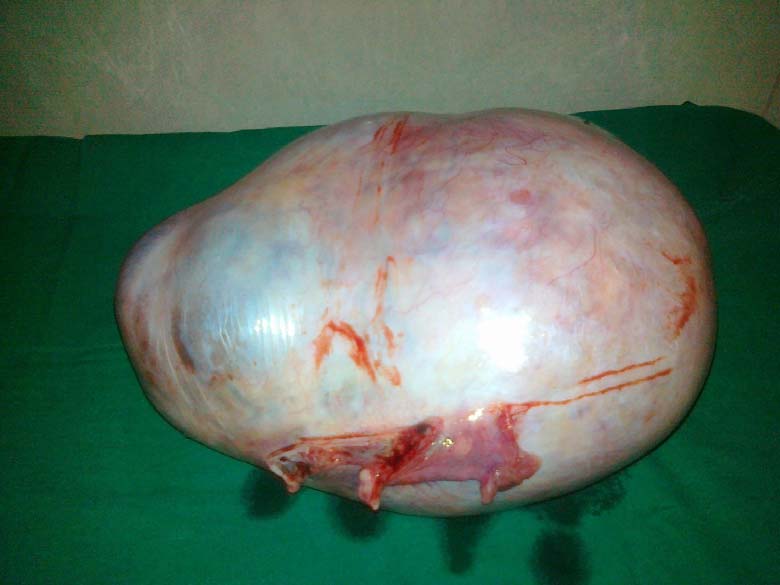
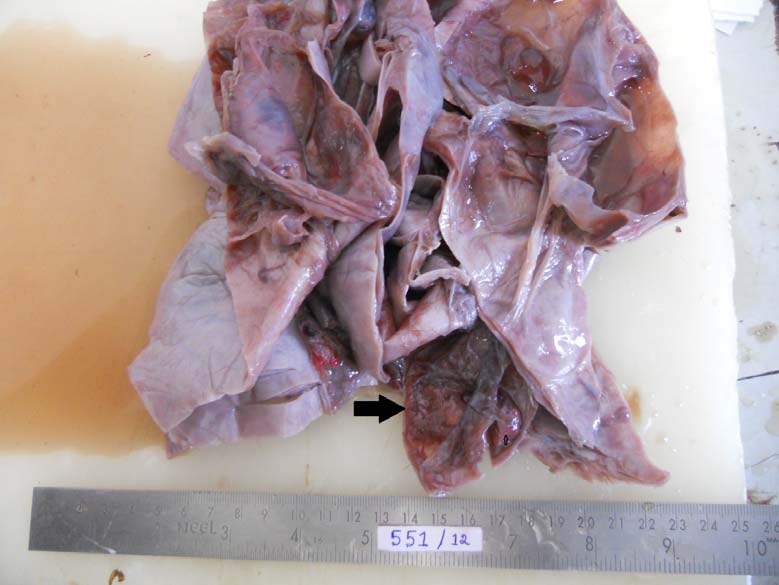
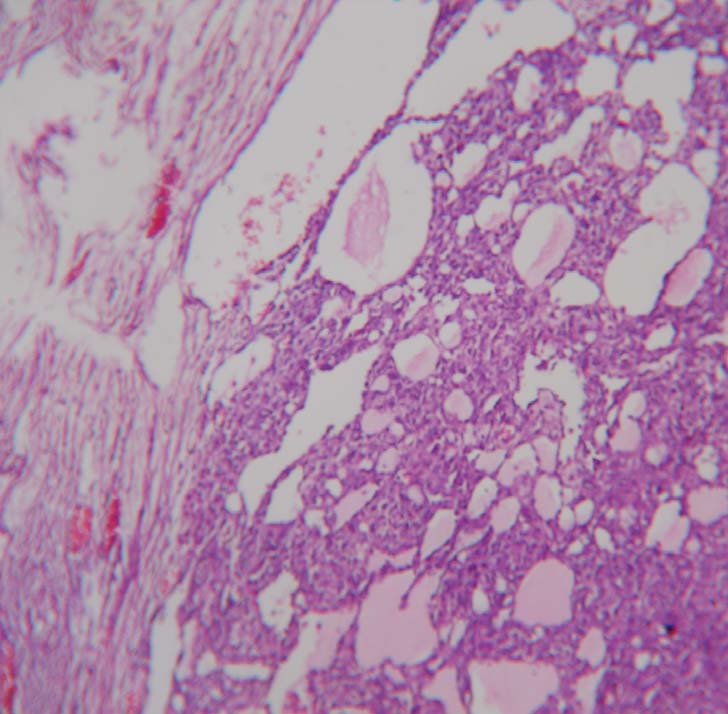
On exploratory laparotomy there was no free fluid. Large cystic tumour was confirmed to arise from and replacing the right ovary. The capsule was thick and smooth [Table/Fig-1]. All the other organs, omentum and peritoneum were normal. The left ovary appeared normal. Right ovariotomy was done. The postoperative period was uneventful. The tumour was mutiloculated containing yellow fluid and gelatinous myxoid areas. Outer wall was smooth.Focal areas showed thickened septa with grey white areas [Table/Fig-2]. Microscopic sections showed predominantly cystic ovarian tumour. The tumour cells were arranged in macrofollicular pattern and solid sheets. The follicular cells showed hyperchromasia and mild atypia with mitotic figures of 4/10 HPF. Occasional tumour cells showed nuclear grooving. The lumen contained eosinophilic mucinous secretion. Some foci showed extensive theca cells. Based on these microscopic findings it was reported as Juvenile Granulosa Cell Tumour [Table/Fig-3].
The patient got her next period spontaneously after 1 month and has been having spontaneous periods within 6-8 weeks. The left ovary is being followed by ultrasound imaging to rule out occurrence of similar tumour.
Large tumour with thick capsule and smooth outer surface
Cut section of the tumour showing multiple loculae and septum with arrow showing thick septum and grey solid areas
Photomicrograph showing predominantly cystic tumour with macrofollicular pattern, eosinophilic mucinous secretion and extensive theca cells. (10x)
Discussion
Anovulation and hirsutism in an adolescent, merits evaluation to rule out hypothyroidism, ovarian tumour and adrenal problems as the underlying causes. Typically, a normal TT level and ultrasonographic imaging of ovaries are sufficient to rule out ovarian tumour and initiate therapy. Granulosa cell tumour can cause hirsultism and anovulation [1]. An ovarian tumour in a case of polycystic ovarian disease developing as a consequence of treatment with insulin sensitizers is rare. It has been reported earlier only by Spremovi-Radjenovi et al [2]. Similar to our case, in their case also an adolescent girl with established diagnosis of PCOD was found to develop ovarian tumour after two years of treatment. In their case it was sertoli leydig cell tumour. They had done a laparoscopy at the outset to rule out ovarian tumour and confirm the diagnosis.
Brisigotti and colleagues [3] have suggested that insulin at high concentration acts via a mechanism mediated by insulin-like growth factor I receptors and could result in the formation of these tumours. Stage I cases in younger age-group carry good prognosis with unilateral salpingo-oopherectomy as the only treatment. Most of the patients with Stage I disease do not require adjuvant therapy.
Conclusion
Patients with PCOS may develop Ovarian tumour after they are initiated on treatment. Ours is probably the third such case reported. Close follow up of the patient is essential for early detection and management of such cases.
[1]. L Vera, M Accornero, M Mora, M Valenzano-Menada, F Minuto, M Giusti, Increasing hirsutism due to a granulosa-cell tumour in a woman with polycystic ovary syndrome: case report and review of the literature 2013 Jan 18 [Epub ahead of print] [Google Scholar]
[2]. S Spremovi-Radjenovi, A Radosavljevi, S Petkovi, S Matijasevi, M Cvetkovi, G Lazovi, The polycystic ovary syndrome associated with ovarian tumour. [Article in Serbian ].Srp Arh Celok Lek 1997 125:375-7. [Google Scholar]
[3]. M Brisigotti, G Fabbretti, F Pesce, R Gatti, A Cohen, G Parenti, Congenital bilateral juvenile granulosa cell tumour of the ovary in leprechaunism: a case report.Pediatr Pathol 1993 13(5):549-58. [Google Scholar]