Biotechnological Production of Inducible Defense-Related Proteins in Edible Radish (Raphanus Sativus) Found in Nepal
Praval Khanal1, Anil Karmacharya2, Shishir Sharma3, Ashwini K. Nepal4, Kanti Shrestha5
1 Student, Department of Biotechnology, Himalayan Whitehouse College of Science and Engineering, Lalitpur, Nepal.
2 Student, Department of Biotechnology, Himalayan Whitehouse College of Science and Engineering, Lalitpur, Nepal.
3 Lecturer, Department of Biotechnology, Himalayan Whitehouse College of Science and Engineering, Lalitpur, Nepal.
4 Senior Demonstrator, Department of Biochemistry, B.P. Koirala Institute of Health and Sciences, Dharan, Nepal.
5 Senior Scientist, National Academy of Science and Technology, Lalitpur, Nepal.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Praval Khanal, Student, Department of Biotechnology, Himalayan Whitehouse College of Science and Engineering, Khumaltar, Lalitpur, Bhaktapur, Nepal.
Phone: +9779841528705,
E-mail: praval.khanal@gmail.com
Background: Fungal infection in plant leads to use of many hazardous antifungal chemicals. Alternative to these chemicals, defense related antifungal proteins can be used in case of fungal diseases.
Aims: An experiment was done in two varieties of edible radish (Raphanus sativus var. Pyuthane Raato and Raphanus sativus var. all season) with aims to produce defense protein within the plant, to identify and perform molecular characterization of those antifungal proteins. The next aim was to compare the antifungal property of those proteins with commercially available synthetic pesticides.
Methods: Both varieties of radish were infected with fungi (Alternaria alternata and Fusarium oxysporum). Protein samples were isolated from leaves following the standard protocol as described for β-glucuronidase (GUS) assay and were run along with the standard protein marker of 10-250kDa in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) to identify and molecularly characterize them.
Results: An additional band in the range of 37-50kDa was observed in the fungal infected samples, which was not seen on uninfected samples. The antifungal assay was carried out for every sample in 96 wells microtitre plate. The extracted protein samples from fungal inoculated plants showed the significant inhibition of fungal growth compared to other samples. On the basis of molecular weight and their antifungal properties, the protein samples from the fungal infected plant were found to be PR2 (Glucanase) and PR3 (Chitinase).
Conclusion: Defense related proteins were successfully produced in two varieties of radish found in Nepal. The use of such biologically produced proteins may reduce the use of biologically harmful synthetic pesticides.
Antifungal protein, Chitinase, Glucanase, Molecular characterization, Raphanus sativus
Introduction
Many chemical fungicides and pesticides are being used in agriculture for the remedy and protection of important food crops from fungal diseases. Several adverse impacts on human health and other parts of ecosystems are attributed to the use of such chemicals [1]. In acute form occurrence of rashes, nausea, vomiting, abdominal pain and eye burn had been reported among many problems, after the human exposure to chemical pesticides [1,2]. However, long-term exposure to chemical pesticides may cause serious illness like- different forms of cancers, neurological disorders like dementia and parkinsonism and serious respiratory disorders [1]. Chronic exposures to such chemicals are known to cause infertility in men and teratogenecity in pregnant women [1,3–4]. Therefore, replacement of such pesticides has been the major concern for recent days [5–9]. New strategies for the protection of crops against microbes are centered round the utilization of natural defense systems against these pathogens that are already present in plants. These natural defense systems present in plants, which are preformed and induced, have been utilized these days for the constant battle for supremacy between pathogens and hosts. Plants are often exposed to their potential microbial pathogens but infection and the subsequent establishment of disease is not so frequent.
The interaction of a plant pathogen with its host can follow two routes; the interaction can either be compatible resulting in a disease; or be incompatible, activates its defense systems to inhibit infection or limit the spread and damage of an established pathogen [10]. In preformed defense system, both chemical and biochemical compounds are accumulated at basal levels within peripheral plant tissue to form microbial barriers and are most prevalent in nutrient-rich plant organs as flower and seeds [11–14]. Similarly, induced defense system relies upon recognition of plant pathogen and elucidation of defense mechanisms activated by a signaling cascade. Early events in the induced defense usually involve hypersensitive cell-death and activation of structural defense [15–17]. One of the most important mechanisms of plant defense is the production of proteinaceous compounds with antimicrobial activity in response to pathogen attack [18–21].
The present study was designed to study the induced defense system of two varieties of edible radish from Nepal, namely- Raphanus sativus, var. Pyuthane raato and Raphanus sativus var. all season, which was carried out with fungi Alternaria alternata and Fusariumoxysporum, as pathogens. Pathogenesis Related Proteins (PRPs) were newly produced on Raphanus sativus upon fungal attack due to the host-pathogen interaction. These antifungal proteins were characterized on the basis of its molecular weight and fungal growth inhibition assay in microtitre plate.
Methodology
Isolation of pure culture of fungi
Potato Dextrose Agar (PDA) (Himedia, India) was prepared on petriplates and subjected to plate exposure on field. A mixed culture of fungi was observed after 5 days of incubation at 25°C. Microscopic and macroscopic studies were performed along with subsequent subculture of each strain for obtaining pure culture of Alteraria alternata and Fusarium oxysporum. Macroscopic study was done on the basis of its colony, which gave woolly to cottony, flat spreading colonies while microscopic study was done by staining the fungal spores with lactophenol cotton blue.
Growing of Raphanus sativus and infection on its leaves with fungal suspension
Raphanus sativus var. Pyuthane Raato and Raphanus sativus var. all season, seeds collected from Annapurna Seed Centre, Kathmandu, Nepal were grown on the soil in simple plastic trays. The soil used for growing both varieties of Raphanus sativus was prepared on the tray by mixing the soil and compost in 3:1 ratio. Some of the Raphanus sativus varieties were subjected to inoculation with 5 drops (5 microlitre) of fungal suspension of 8 x 105 cells/ml of fungal culture in their leaves after injuring them at their leaf veins.
Protein extraction and Sodium Dodecyl Sulfate PolyAcrylamide Gel Electrophoresis (SDS-PAGE)
Raphanus sativus leaves were collected after incubation for 3 days and were grinded by mortars and pestles in QB buffer (100mM potassium phosphate buffer pH 7.8, 1 mM of EDTA, 1% Triton-X 100 and 10% glycerol with several protease inhibitors). Protein was extracted from them using β-glucuronidase (GUS) method. The isolated protein samples were run on SDS-PAGE during electrophoresis along with standard protein marker molecular weight (BIO-RAD) ranging from 10kDa-250kDa. Non infected samples were also run in Polyacrylamide Gel Electrophoresis (PAGE).
Antifungal Assay
Antifungal assays against the introduced fungi, Alternaria alternata and Fusarium oxysporum were carried out in 96 wells microtitre plate with isolated fungal infected and non infected protein samples. Fungal cell suspension concentration of 103 cells/ml was used for the antifungal assay. The absorbance of all the samples were taken by the microplate reader set at 415nm after 20 hours and 48 hours. 0.1% Copper oxychloride (fungicide) was used as positive control, infected samples as test samples and non infected samples as negative control. Each of the analysis was performed on quadruplicate.
Results
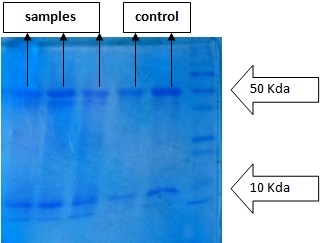
Different bands were observed in SDS-PAGE after staining and visualization step. Each bands were found to be in between 10kDa and 50kDa when samples were run in PAGE along with standard protein marker (BIO-RAD, India) ranging from 10-250kDa. An additional protein band was observed in samples from fungal infected Raphanus sativus in range of 37-50kDa [Table/Fig-1,2].
Standard Protein Marker (10-250) kDa
SDS-PAGE of fungal infected and non infected protein samples with standard protein marker (10-250) kDa
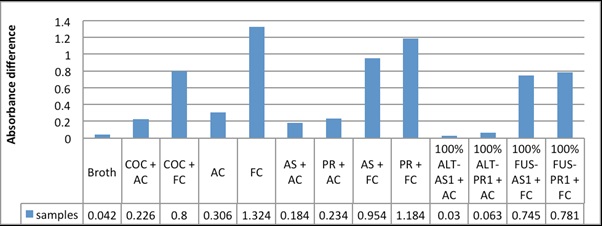
During antifungal assay, the protein samples from the fungal infected Raphanus sativus plants significantly inhibited the fungal growth, than that from the non infected plants. Copper oxychloride (0.1%), commercially available fungicide showed less antifungal action when compared with samples isolated along with the antifungal protein produced by infected plants [Table/Fig-3,4&5].
Absorbance difference graph between the initial and final reading of different samples on 96-well microtitre plate after 48 hours of samples addition on fungal culture ,after microtitre plate
Growth of the fungus in each samples with respect to time
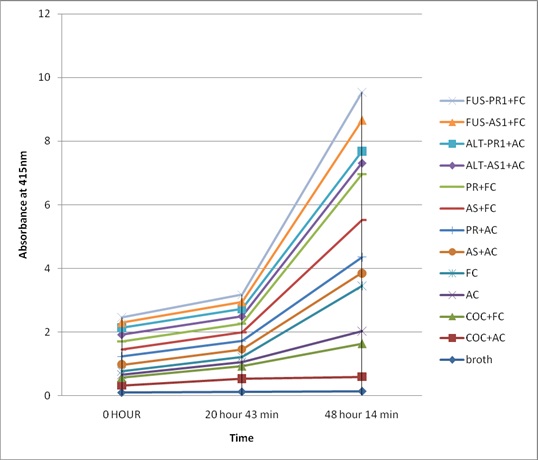
Absorbance reading of different samples at different time at 415 nm
| Samples | Absorbance at 415 nm |
|---|
| 0 HOUR | 20 hours 43 minutes | 48 hours 14 minutes |
| Broth | | | | | 0.101 | | | | | 0.126 | | | | | 0.143 |
| COC + AC | | | | | 0.228 | | | | | 0.409 | | | | | 0.454 |
| COC + FC | | | | | 0.235 | | | | | 0.387 | | | | | 1.035 |
| AC | | | | | 0.099 | | | | | 0.139 | | | | | 0.405 |
| FC | | | | | 0.1 | | | | | 0.153 | | | | | 1.424 |
| AS + AC | | | | | 0.214 | | | | | 0.242 | | | | | 0.398 |
| PR + AC | | | | | 0.255 | | | | | 0.263 | | | | | 0.489 |
| AS + FC | | | | | 0.219 | | | | | 0.272 | | | | | 1.173 |
| PR + FC | | | | | 0.256 | | | | | 0.267 | | | | | 1.44 |
| 100% ALT-AS1 + AC | 0.218 | 0.205 | 0.219 | 0.213 | 0.21375 | 0.257 | 0.225 | 0.239 | 0.239 | 0.24 | 0.248 | 0.425 | 0.275 | 0.476 | 0.356 |
| 100% ALT-PR1 + AC | 0.208 | 0.209 | 0.209 | 0.215 | 0.21025 | 0.321 | 0.167 | 0.209 | 0.206 | 0.22575 | 0.271 | 0.155 | 0.434 | 0.596 | 0.364 |
| 100% FUS-AS1 + FC | 0.173 | 0.159 | 0.15 | 0.202 | 0.171 | 0.241 | 0.213 | 0.202 | 0.254 | 0.2275 | 0.918 | 0.979 | 1.029 | 0.977 | 0.97575 |
| 100% FUS-PR1 + FC | 0.151 | 0.164 | 0.149 | 0.163 | 0.15675 | 0.194 | 0.207 | 0.187 | 0.308 | 0.224 | 0.932 | 1.148 | 0.965 | 0.456 | 0.87525 |
COC: Copper oxychloride, AC: Alternaria Culture, FC: Fusarium Culture, AS: Raphanus sativus var all season, PR: Raphanus sativus Pyuthane Raato, ALT-AS1: Alternaria infected Raphanus sativus var all season, ALT-PR1: Alternaria infected Pyuthane Raato, FUS-AS1: Fusarium infected Raphanus sativus var all season, FUS-PR1: Fusarium infected Pyuthane Raato
The inhibition of the fungal culture by the inducible antifungal proteins with inherent proteins and fungal uninfected samples were compared at different time intervals (20 hours and 48 hours of incubation with protein samples). Similarly, the antifungal property of 0.1% copper oxychloride was also studied. The comparative study showed that the proteins with inducible antifungal proteins showed high antifungal property against the fungi Alternaria alternata and Fusarium Oxysporum compared to proteins isolated from the uninfected radish and 0.1% copper oxychloride [Table/Fig-4,5].
Discussion
The present study was conducted in two varieties of radish (infected and non-infected), in order to produce natural antifungal proteins, identify them and compare antifungal activity of such proteins with the commercially available antifungal chemical. Proteins from both varieties of radish (both: infected and non-infected) were isolated and were run in the gel of SDS-PAGE along with protein marker. Different protein bands were observed between 10kDa-50kDa in the gel of SDS-PAGE. This finding suggests that entire Raphanus sativus has some inherent proteins which were produced during their lifecycle. An additional protein band on the range of 37-50kDa was observed in protein samples isolated from Raphanus sativus plants infected with fungi, while compared with non-infected one. It indicates that there is expression of new protein when Raphanus sativus is subjected to infection by fungi, Alternaria alternata and Fusarium oxysporum, which are also considered as one of the important biotic stresses for Brassicaceae crops like Raphanus sativus that may cause leaf spot.
The antifungal assay in microtitre plate of the extracts (fungal infected and non-infected Raphanus sativus samples) and chemical fungicide (0.1% Copperoxychloride) showed different results. When antifungal assay of these extracted proteins were carried out, the difference in the initial and final reading was high in broth culture of Alternaria and Fusarium than the infected samples. This finding depicts that the Raphanus sativus protein isolated from the plants infected with these fungi inhibit the growth of these fungi.
The combined results of SDS-PAGE and antifungal assay of the isolated proteins showed that the new protein of the range 37-50kDa was responsible for the inhibition of the growth of the fungi. It is known that the molecular weight of chitinase and glucanase lie in this protein range which is responsible for the inhibition of fungi (by dissolving its chitin and glucagon component of cell wall). Both of these proteins belong to PR2 and PR3 family of antifungal protein [22–25]. PR2 proteins have (1,3) β-endoglucanase activity which hydrolyze the structural 1,3-β glucagon present in fungal cells. This weakens the cell wall and the weakened cell wall results in cell lysis and death [22]. Similarly, chitinases cleave cell wall chitin polymers resulting in a weakened cell wall and rendering fungal cells osmotically sensitive [25]. Not surprisingly, PR2 and PR3 proteins act synergistically in inhibiting fungal growth, both in vitro and in plants [23]. This showed that the biotechnologically produced defense-related proteins from edible radish (Raphanus) sativus) found in Nepal possessed better antifungal properties than synthetic chemical pesticides. Thus, use of chemical pesticides can be minimized if these natural defense proteins are used against fungal diseases in Raphanus sativus and other plants, which avoids people from exposure to potential health risks to farmers associated with such chemicals. In addition to this, the production of the natural antifungal proteins also minimizes the chances of potential mycotoxicoses in the people who consume these edible plants, vegetables and fruits. If this can be practiced, many issues and incidences related to the harmful effects of pesticides will be significantly decreased.
Limitations
The experimental condition for both the fungal infected and non-infected Raphanus sativus was identical. But temperature, humidity and other parameters were changing according to natural condition.
Limited numbers of Raphanus sativus varieties, fungi and chemical fungicide were taken into account.
Antifungal proteins on the range of 10 to 250kd were identified. Any antifungal protein beyond this level weren’t identified due to narrow range of protein marker.
Antifungal proteins were just identified and antifungal assay was carried along with other inherent proteins the Raphanus sativus produced during their life cycle. Pure antifungal protein was not isolated so the antifungal assay seen was the result of the difference between the activity of inherent proteins and the inherent proteins along with the antifungal protein produced.
Conclusion
The present study successfully evaluated the role of antifungal proteins produced in Raphanus sativus var. Pyuthane Raato and Raphanus sativus var all season after the exposure to biotic stress, fungi, Alternaria alternata and Fusarium oxysporum. Chitinase (PR3) and Glucanase (PR2) proteins of the molecular weight ranging between 37-50kDa were produced in Raphanus sativus varieties after the infection with these fungi. The produced chitinase and glucanase possessed good antifungal properties in comparison to commercially available pesticide (0.1% copperoxychloride). Thus, if this process is applied in real field, biologically synthesized antifungal proteins can serve as alternatives to the hazardous chemical pesticides.
COC: Copper oxychloride, AC: Alternaria Culture, FC: Fusarium Culture, AS: Raphanus sativus var all season, PR: Raphanus sativus Pyuthane Raato, ALT-AS1: Alternaria infected Raphanus sativus var all season, ALT-PR1: Alternaria infected Pyuthane Raato, FUS-AS1: Fusarium infected Raphanus sativus var all season, FUS-PR1: Fusarium infected Pyuthane Raato
[1]. Sanborn M, et al. Systematic review of pesticide health effects. Ontario: Ontario College of Family Physicians. Available at: http://www.ocfp.on.ca/docs/pesticides-paper/2012-systematic-review-of-pesticide.pdf [Accessed on 01/07/2013] [Google Scholar]
[2]. Gupta P, Pesticide exposure—Indian sceneToxicology 2004 198(1):83-90. [Google Scholar]
[3]. García AM, Pesticide exposure and women’s healthAmerican journal of industrial medicine 2003 44(6):584-94. [Google Scholar]
[4]. Rupa D, Reddy P, Reddi O, Reproductive performance in population exposed to pesticides in cotton fields in IndiaEnvironmental research 1991 55(2):123-28. [Google Scholar]
[5]. Elad Y, Mechanisms involved in the biological control ofBotrytis cinerea incited diseasesEuropean Journal of Plant Pathology 1996 102(8):719-32. [Google Scholar]
[6]. Elad Y, Shtienberg D, Botrytis cinerea in greenhouse vegetables: chemical, cultural, physiological and biological controls and their integrationIntegrated Pest Management Reviews 1995 1(1):15-29. [Google Scholar]
[7]. Köhl J, Biological control of Botrytis cinerea in cyclamen with Ulocladium atrum and Gliocladium roseum under commercial growing conditionsPhytopathology 1998 88(6):568-75. [Google Scholar]
[8]. McLaughlin R, Biological control of postharvest diseases of grape, peach, and apple with the yeasts Kloeckera apiculata and Candida guilliermondiiPlant disease 1992 76(5):470-73. [Google Scholar]
[9]. Tronsmo A, Ystaas J, Biological control of Botrytis cinerea on apple [by Trichoderma harzianum]Plant Diseases 1980 :64 [Google Scholar]
[10]. Bell JN, Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interactionMol Cell Biol 1986 6(5):1615-23. [Google Scholar]
[11]. Broekaert WF, Plant defensins: novel antimicrobial peptides as components of the host defense systemPlant physiology 1995 108(4):1353 [Google Scholar]
[12]. Ferreira RB, The role of plant defence proteins in fungal pathogenesisMolecular Plant Pathology 2007 8(5):677-700. [Google Scholar]
[13]. Osbourn AE, Preformed antimicrobial compounds and plant defense against fungal attackThe Plant Cell 1996 8(10):1821 [Google Scholar]
[14]. Stahl EA, Bishop JG, Plant–pathogen arms races at the molecular levelCurrent opinion in plant biology 2000 3(4):299-04. [Google Scholar]
[15]. Devlin WS, Gustine DL, Involvement of the oxidative burst in phytoalexin accumulation and the hypersensitive reactionPlant physiology 1992 100(3):1189-95. [Google Scholar]
[16]. Greenberg JT, Yao N, The role and regulation of programmed cell death in plant–pathogen interactionsCellular microbiology 2004 6(3):201-11. [Google Scholar]
[17]. Kiraly L, Barna B, Király Z, Plant resistance to pathogen infection: forms and mechanisms of innate and acquired resistanceJournal of Phytopathology 2007 155(7–8):385-96. [Google Scholar]
[18]. Ahn I-P, Park K, Kim C.-H., Rhizobacteria-induced resistance perturbs viral disease progress and triggers defense-related gene expressionMolecules and cells 2002 13(2):302 [Google Scholar]
[19]. Koga J, Cholic acid, a bile acid elicitor of hypersensitive cell death, pathogenesis-related protein synthesis, and phytoalexin accumulation in ricePlant physiology 2006 140(4):1475-83. [Google Scholar]
[20]. Nürnberger T, Innate immunity in plants and animals: striking similarities and obvious differencesImmunological reviews 2004 198(1):249-66. [Google Scholar]
[21]. Van Loon LM, Rep M, Pieterse C, Significance of inducible defense-related proteins in infected plantsAnnu. Rev. Phytopathol 2006 44:135-62. [Google Scholar]
[22]. Beffa Meins F Jr, Pathogenesis-related functions of plant β-1, 3-glucanases investigated by antisense transformation—a reviewGene 1996 179(1):97-103. [Google Scholar]
[23]. Jach G, Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobaccoThe Plant Journal 1995 8(1):97-109. [Google Scholar]
[24]. Van Loon L, Pathogenesis-related proteinsPlant Molecular Biology 1985 4(2):111-16. [Google Scholar]
[25]. Watanabe T, Family 19 chitinases of Streptomyces species: characterization and distributionMicrobiology 1999 145(12):3353-63. [Google Scholar]