We report a case of a preterm neonate with isolated spontaneous pneumopericardium occurring in the absence of a history of neonatal resuscitation, mechanical ventilation, major lung disease or another air leak. The pneumopericardium was asymptomatic and resolved without the need for aspiration, although the “nitrogen washout” technique was used.
Pneumopericardium, Preterm, Respiratory Distress Syndrome (RDS)
Introduction
Pneumopericardium (PPC) in the neonatal period is a rare clinical condition which is usually associated with other simultaneously-occurring air leaks. The majority of reported cases are of preterm newborns with Respiratory Distress Syndrome (RDS) who had required active positive pressure during resuscitation and/or subsequent respiratory support, whether in the form of mechanical ventilation or Continuous Positive Airways Pressure (CPAP).
There have been some reported cases of spontaneous PPC in term neonates who had not received mechanical ventilation or active resuscitation at birth. We report case, of a preterm newborn with isolated spontaneous PPC occurring in the absence of a history of neonatal resuscitation, mechanical ventilation, lung disease or another air leak.
Case Report
A pre-term male newborn was born at 34 weeks gestation to a 37 year old Caucasian primigravid woman who presented to delivery suite for threatened premature labour and a small antepartum haemorrhage. The pregnancy was complicated by premature pre-term rupture of membranes since 23 weeks of gestation for which she received erythromycin prophylaxis. She had received a full course of antenatal steroids at 28 weeks of gestation.
The baby was vertex and delivered spontaneously by vaginal route. He cried immediately after birth and did not require any resuscitation. He had a birth weight of 2.585 kg, a length of 47 cm and a head circumference of 34.5 cm (all around the 90th centile for age).
The baby developed mild respiratory distress soon after birth. He was transferred to the Neonatal Intensive Care Unit (NICU) in view of both his prematurity and the respiratory distress. During transfer he received facemask oxygen with no CPAP.
On admission his vital parameters showed a temperature of 36.1°Celsius, heart rate of 170/minute and a respiratory rate of 60/minute. He was grunting with subcostal and intercostal recession as well as nasal flaring. Blood pressure measured with automatic oscillatory method was 64/52mmHg (mean 56 mmHg); capillary refill on admission was 5 seconds. Chest examination showed subcostal and intercostal retractions; on auscultation air entry was audible and bilaterally equal. Cardiovascular examination was normal with normal heart sounds, no audible murmur and normal femoral and peripheral pulses.
On arrival to the NICU he was commenced on crib oxygen with a FiO2 of 0.4. At 1 hour of age he had a sudden episode of apnoea and bradycardia, during which he become pale, dusky and clinically cyanosed. He responded to tactile stimulation. His vital parameters showed a temperature of 36.9° Celsius, heart rate of 145/minute, respiratory rate of 66/minute with grunting, subcostal and intercostal recession and nasal flaring. Blood pressure measured with automatic oscillatory method was normal; capillary refill was 3 seconds.
His physical examination at this time showed marked respiratory distress. Heart examination showed normal heart sounds with no audible murmur and normal pulses. He was initially given 100% facemask oxygen for 5 min, to which he responded well. His FiO2 was subsequently changed from the initial 0.40 to 0.7. His capillary blood gas showed pH 7.276, pO2 50.4mmHg, pCO2 53.6mmHg, base excess –3.3 and lactate of 1.3.
Chest X-ray at 2 hours of age showed a PPC with no evidence of any other air leak [Table/Fig-1].
Chest X-ray showing the characteristic “halo” sign of a pneumopericardium
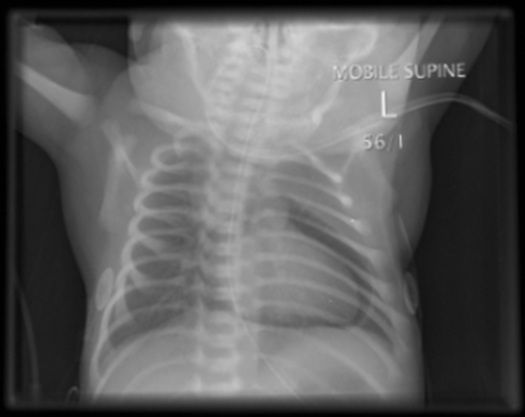
After review of the chest X-ray, his FiO2 was increased to 1.0 so as to facilitate nitrogen washout. As there were no signs of cardiac tamponade, aspiration of this PPC was not attempted and the baby was observed with continuous monitoring of cardio-respiratory status, transcutaneous gases, pulse oximetry, four-hourly automatic oscillatory blood pressure monitoring and regular capillary gases.
The infant’s clinical condition improved and his respiratory distress settled quickly. His chest X-ray 5 hours later showed a complete resolution of the PPC. In view of clinical and radiological improvement, the oxygen was gradually weaned to room air by 18 hours of age. His capillary gas in room air was normal.
Case Discussion
In the largest reported group, a 13-year (1977-1989) retrospective cohort group analysis 1 of 47 newborns with PPC, the incidence of neonatal PPC in very low birth weight (VLBW, ≤1.5kg) newborn admissions overall was 2%. In this same group, the incidence was 3.5% of the ventilated VLBW cohort, none of which had received surfactant. PPC is more common in male infants [1]. The majority of reported cases are preterm low birth weight infants having required some degree of respiratory support for RDS [2–5].
PPC is usually associated with other simultaneously-occuring air leaks, such as pneumothorax (49%) [1], pulmonary interstitial emphysema (46%) [1], pneumomediastinum (39%) [1], subcutaneous emphysema (17%) [1], pneumoperitoneum (10%) [1] and systemic vascular air embolus (5%) [1].
There are a number of published cases of spontaneous isolated PPC in term neonates who had not received mechanical ventilation or active resuscitation at birth [6–8].
In Hook et al., cohort [1]; of VLBW ventilated newborns, the mean age at occurrence of a PPC was 3.3 days (median 1.25 days, range 0-34 days). Majority of late onset [3–5] (3 out of 4) required active intervention. All three cases [6–8] of early onset including the current one dose not required any active intervention.
The exact pathophysiology of neonatal pneumopericardium is still unclear. Air is thought to dissect from ruptured alveoli along the perivascular sheaths to the hilum and mediastinum, with subsequent rupture into the thorax or mediastinum [9]. Rupture of this air into the pericardium has been postulated to occur in a possible anatomical area of weakness at the reflection of the parietal and visceral pericardium near the area of the pulmonary veins [9].
Chest X-ray is the standard diagnostic method. The classic finding in PPC is the “halo sign”: air completely surrounding and outlining the heart but not extending beyond the reflection of the pericardium onto the great vessels [10].
A conservative approach of close monitoring without aspiration may be considered if there are no signs of cardiac tamponade, as in our case. Additional oxygen therapy as a “nitrogen washout” technique has been documented as potentially beneficial in neonatal PPC [11]; this approach would not be appropriate in a newborn < 32 weeks’ gestation at risk of possible retinopathy of prematurity.
To conclude, isolated PPC is rare in neonates when compared to other air leaks, and, like all air leaks, is becoming less common with changes in neonatal care. Asymptomatic PPC without any associated lung pathology or air leaks may resolve with a conservative approach of close monitoring, with or without extra oxygen.
[1]. Hook B, Hack M, Morrison S, Borawski-Clark E, Newman NS, Fanroff A, Pneumopericardium in very low birth weight infantsJ Perinatol 1995 15(1):27-31. [Google Scholar]
[2]. Durward PC, Pneumopericardium in a neonateAustralas Radiol 1966 10:229-30. [Google Scholar]
[3]. Gershanik JJ, Neonatal pneumopericardiumAm J Dis Child 1971 121:438-39. [Google Scholar]
[4]. Singh KR, Wiglesworth FW, Stern L, Pneumopericardium in the newborn - a complication of respiratory managementCan Med Assoc J 1972 106:1195-96. [Google Scholar]
[5]. Brans YW, Pitts M, Cassady G, Neonatal pneumopericardiumAm J Dis Child 1976 47:634-35. [Google Scholar]
[6]. Rhodes PG, Berry PL, Goodwin CC, Pneumopericardium in a neonate not artificially ventilatedArch Dis Child 1980 55:164-65. [Google Scholar]
[7]. Björklund L, Lindroth M, Malmgren N, Warner A, Spontaneous pneumopericardium in an otherwise healthy full-term newbornActa Pediatr Scand 1990 79:234-36. [Google Scholar]
[8]. Itani MH, Mikati MA, Early onset neonatal spontaneous pneumopericardiumJ Med Liban 1998 46(3):165-67. [Google Scholar]
[9]. Mansfield PB, Graham CB, Beckwith JB, Pneumopericardium and pneumomediastinum in infants and childrenJ Pediatr Surg 1973 8:691-98. [Google Scholar]
[10]. Burt TB, Lester PD, Neonatal pneumopericardiumRadiology 1982 142:81-84. [Google Scholar]
[11]. Lawson EF, Gould JB, Taeusch HW Jr, Neonatal pneumopericardium: current managementJ Pediatr Surg 1980 15(2):181-85. [Google Scholar]