Oxidative stress is a condition in which oxidant metabolites exert their toxic effect because of an increased production or an altered cellular mechanism of protection [7]. Increased oxidative stress and the generation of the free oxygen radicals can result in modification of LDL to oxidized LDL that could lead to atherosclerotic lesions, which is central to the genesis of AMI [8]. Normally, there is a balance between tissue oxidant and antioxidant activity. The latter is achieved by the antioxidant scavenger system, which includes enzymes like SOD, catalase, GPx. When there is an excessive addition of oxidants from exogenous sources added to the endogenous production, the available tissue defense system becomes overwhelmed resulting in oxidative damage to the tissues [9].
Though AMI is extensively studied, the role of obesity as an important risk factor is poorly explained with reference to AMI. This study is aimed to evaluate the relationship between obesity manifested by increased WHR ratio and oxidative stress in patients of AMI.
Methods
Study design: The study consisted of 60 patients (41 men and 19 women) with a mean age of 49.50 ± 6.28 years admitted in the Coronary Care Unit of Jawaharlal Nehru Medical College Hospital, Aligarh Muslim University, Aligarh, Uttar Pradesh, India with the diagnosis of AMI. The diagnosis of AMI was based on a history of prolonged ischemic chest pain, which lasted for up to 3 hours, ECG changes (ST elevation of 2 mm or more in at least two leads) and elevated creatine kinase isoenzyme MB (CK-MB) and troponin T within 12 hours after the onset of pain. The control group consisted of 60 healthy, age-matched subjects, 45 men and 15 women, recruited from the institution. The study was duly approved by the Board of Studies/Institutional Ethical Committee and a valid and informed consent was obtained from all the subjects (including both case and control) of our study.
Inclusion criteria: Patients with diagnosis of AMI and admitted within 24 hours of onset of symptom.
Exclusion criteria: Patients/Control with any history of diabetes mellitus, asthma, smoking, oral antioxidant or vitamin intake.
Anthropometry
A digital scale, with an accuracy of ±100 g, was used to measure body weight (BW). Standing body height (BH) was measured to the nearest 0.5 cm with a commercial stadiometer without shoes with the shoulders in a relaxed position and the arms hanging freely. WC was measured, at the level midway between the lower rib margin and the iliac crest. HC was measured at the fullest point around the buttocks. WC (cm) was divided by HC (cm) to calculate WHR. Normal reference value for WRH is <0.85 in females and <0.95 in males.
Blood Collection and Biochemical Methods
Five ml of blood sample was drawn under aseptic condition from the peripheral vein of the subjects of both the sexes. It was centrifuged at 3,000 rpm for 15 minutes. The serum was subjected to estimation of MDA antioxidants namely GPx and SOD.
Assay of SOD
SOD was determined by method of McCord and Fridovich [12]. Superoxide was generated in a system comprising NADH and Phenazine Methosulphate. These superoxide anions reduced the nitroblue tetrazolium, forming a blue formazone, which was measured at 560 nm. SOD inhibited reduction of NitroblueTetrazolium and thus, enzyme activity was measured by monitoring the rate of decrease in optical density at 560 nm. Enzyme activity was expressed as units per milligram of serum protein. The protein content of serum was measured by the method put forth by Lowry et al., [13].
Assay of GPx
The glutathione peroxidase activity was determined by the procedure of Paglia et al., [14]. Briefly, the oxidized glutathione produced during GPx enzyme reaction was immediately reduced by NADPH and glutathione reductase. Therefore, the rate of NADPH consumption was monitored as a measure of formation of oxidized glutathione. Results were expressed as nanomole of NADPH Oxidized per minute per milligram serum Protein.
Assay of MDA
MDA levels were estimated by thiobarbituric acid (TBA) reaction. Using 40% trichloroacetic acid, proteins were precipitated from 0.5 ml serum and precipitated proteins were incubated with TBA reagent in a boiling water bath for 60 minutes. The colored complex that occurred was refrigerated to room temperature and measured by using a spectrophotometer at 533 nm.1,1,3,3-5 tetraethoxypropane (1 μmol/L) was used as a standard for MDA estimation. Concentrations of MDA were expressed in μM/l.
Statistical Analysis
The data from patients and controls were compared using Student’s t-test. Pearson’s correlation coefficients were determined between the measured parameters at 5% level of significance. Values were expressed as mean ± standard deviation (SD).Statistical Package for Social Science (SPSS) ver. 17.0 was used for statistical analysis. p-value of less than 0.05 was considered to indicate statistical significance.
Observation and Results
Demographic data of control and AMI group are shown in [Table/Fig-1].
Demographic data, Clinical characteristics and cardiac biomarkers of the healthy group and AMI patients.
| Variables | Control (N=60) | Cases of AMI (N=60) |
|---|
| Age (years) | 46.75 ± 7.69 | 49.50 ± 6.28 |
| Male | 67.5% | 70% |
| Female | 32.5% | 30% |
| Weight (kg) | 60.17 ± 7.73 | 65.80 ± 7.59* |
| Height (cm) | 163.06 ± 6.38 | 163.82 ± 5.93 |
| BMI (Kg/m2) | 22.44 ± 2.09 | 25.02 ± 3.01* |
| Waist-to-hip ratio | 0.84 ± 0.05 | 0.96 ± 0.11* |
| Systolic blood pressure (mm of Hg) | 119.75 ± 7.50 | 132.20 ± 8.51* |
| Diastolic blood pressure (mm of Hg) | 79.85 ± 6.63 | 82.95 ± 6.21* |
| Hypertension | – | 52% |
| CK (IU /L) | 73 ± 15.6 | 126 ± 26.5* |
| CK-MB (IU/ L) | 12.5 ± 2.8 | 97 ± 7.8* |
| Troponin T (ng/ml) | 0.021 ± 0.005 | 1.97 ± 0.14* |
Continuous variables are presented as mean ± SEM and the other variables are shown as percentage of patients.
*Represents ‘p’ value <0.05
Age and sex: AMI group had 41 males and 19 females with mean age 49.50 ± 6.28 years. The control group had 45 males and 15 females with a mean age of 46.75 ±7.69 years.
Height and weight: The mean height of AMI patients was 163.82 ± 5.93 cm and that of control group was 163.06 ± 6.38 cm. AMI patients had a mean weight of 65.80 ± 7.59 kg which was significantly higher than that of controls where the mean weight was 60.17 ± 7.73 kg.
Blood pressure: AMI patients showed a significant rise in both systolic, as well as, diastolic blood pressure as compared to control.
WHR: The mean WHR for the control group was 0.84 ± 0.05. Mean WHR measured in cases of AMI was 0.96 ± 0.11. A significant difference is observed in WHR of control and cases of AMI (p-value = 0.001).
Antioxidant enzymes GPx, SOD and the MDA levels in control and AMI group are shown in [Table/Fig-2].
Antioxidant Status and MDA level in AMI and Control.
| Variables | Control (N=60) | Cases of AMI (N=60) |
|---|
| Superoxide dismutase (SOD) (unita mg/serum protein) | 9.33 ± 0.28 | 8.92 ± 0.06* |
| Glutathione peroxidase (GPx) (unitb mg/serum protein) | 57.16 ± 0.32 | 49.18 ± 0.39* |
| Malondialdehyde (MDA)(μM/l) | 1.21 ± 0.06 | 1.49 ± 0.11* |
a One unit of activity was taken as the enzyme reaction, which gave 50% inhibition of NBT reduction in one minute.
b Nanomole of NADPH Oxidized per minute.
* Represents ‘p’ value <0.05
Both, SOD and GPx were significantly decreased in AMI group (p < 0.001) as compared with controls. MDA levels showed a significant increase in AMI group (p < 0.01) as compared to controls. A negative correlation was observed between rise in MDA and fall in Antio-xidant enzyme levels. WHR showed a positive correlation with MDA level [Table/Fig-3,4&5].
Correlation between WHR the oxidative stress markers
| Parameters | r-value | p-value |
|---|
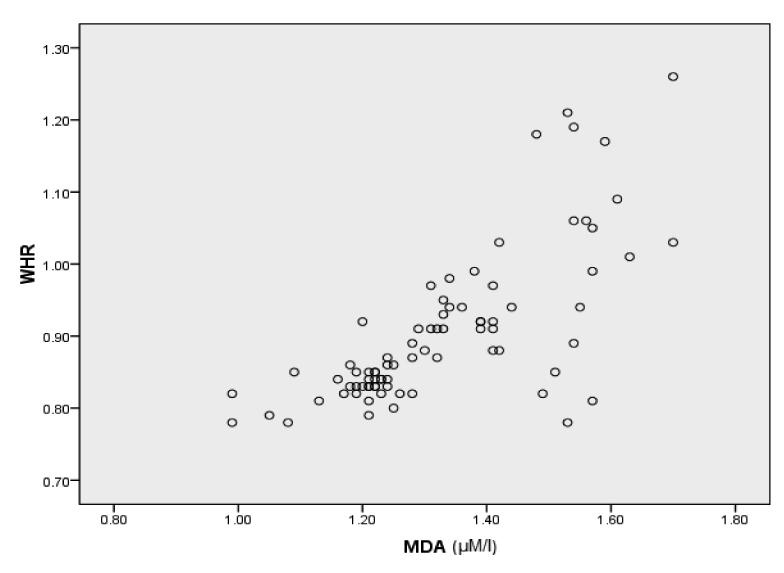
| WHR and MDA | 0.727 | 0.01 |
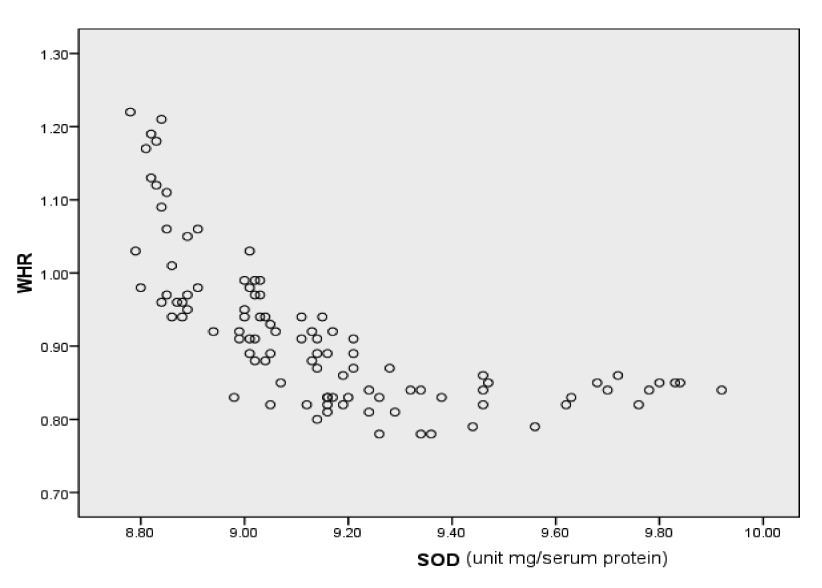
| WHR and SOD | -0.487 | 0.01 |
| WHR and GPx | -0.624 | 0.01 |
Correlation graph of WHR with SOD
Correlation graph of WHR with MDA
Discussion
AMI is the main cause of mortality and a major cause of morbidity and disability worldwide [15]. Atherosclerosis, the most common pathologic process underlying cardiovascular disease, represents a state of heightened oxidative stress characterized by lipid and protein oxidation in the vascular wall [16,17]. Oxidation of low density lipoproteins is considered a key initial step in atherosclerosis and CHD development and progression [18]. Various risk factors have been attributed in the genesis of AMI such as heredity, dyslipidemia, obesity, hypertension and lifestyle stress. In addition to traditional risk factors, oxidative stress has been regarded as one of the most important contributors to the progression of atherosclerosis [19]. Increased lipid peroxidation is thought to be a consequence of oxidative stress, which occurs when the dynamic balance between pro-oxidant and antioxidant mechanism is impaired. It has been suggested that there is increased lipid peroxides levels in blood of patients with AMI [20]. We observed in the study an increased concentration of MDA in the circulation of total AMI patients indicating increased lipid peroxidation. Significant rise of MDA level, a lipid peroxidation product, is indicative of elevated oxidative stress in AMI patients. Increased lipid peroxidation in AMI is explained by the fact that, in AMI patients, because of ischemia there is drastic reduction of ATP, which is converted to hypoxanthine and then to uric acid by xanthine oxidase upon reperfusion. During this process enormous amounts of superoxide radicals are formed which can simulate Haber-Weiss reaction for further generation of ROS, initiating lipid peroxidation [21]. Crisby et al., [22], and Nikolic H et al., [23] found increased levels of MDA in the blood of patients with CHDs and AMI patients treated by percutaneous coronary intervention. A similar study by Eva S et al., [24] showed MDA levels in AMI patients at admission were higher than in control patients (1.66 + 0.55 vs. 1.44 + 0.55 mmol/l) but showed a sustained decrease over the 3 hour after reperfusion of the occluded artery. The findings of the present study confirm the previous studies and demonstrate that patients suffering from AMI exhibit a higher plasma concentration of MDA.
Obesity has been clearly established as an independent risk factor for the development of CAD and AMI [25]. It has been demonstrated that excess body fat is an important risk factor for AMI. Investigators have shown that central obesity or visceral fat, represented by WHR, more accurately reflects abnormal metabolic status than excess fat alone or obesity per se [26]. Visceral adipose tissue is a major site of cytokine production that contributes to atherosclerotic progression. It plays a major role in insulin resistance and dyslipidemia and in the induction of prothrombotic and inflammatory states, which is central to the onset of AMI [27]. In the present study the mean WHR of control group was 0.84 ± 0.05. Mean WHR measured in cases of AMI was 0.96 ± 0.11. A significant difference is observed in WHR of control and cases of AMI (p < 209 0.001). A positive correlation has been found between WHR and MDA level denoting that with increase in obesity there is an increase in the lipid peroxidation. Neela P et al., [28] reported a similar finding in a study conducted on 100 patients of AMI.
A reason for increased lipid peroxidation in plasma of patients AMI may be a poor enzymatic and non-enzymatic antioxidant defense system. SOD along with GPx, the preventive antioxidants, plays a very important role in protection against lipid peroxidation. In this study, SOD and GPx activities were significantly lower in AMI patients than in control subjects. This indicates severe damage to antioxidant system, which is unable to combat oxidative stress and inflammation [29].
Limitation of The Study
The small size of the study and of the control group limited the determination of measured clinical parameters. The results, therefore, can be considered to show only the trend and not any firm conclusion. Nevertheless, the result will justify further research on large sample size.
Conclusion
The present study is suggestive of imbalance between oxidant and antioxidants in AMI patients. The decreased activities of antioxidant enzymes may be a compensatory regulation in response to increased oxidative stress. Obesity reflected by an increased WHR has been clearly demonstrated as an aggravating factor which further increases oxidative stress and disrupts the oxidant-antioxidant balance. Thus, weight reduction may be hypothesized to have an important prognostic value in cases of AMI.
Authors’ Contributions
Anwar H Siddiqui designed the data tool, collected the data and was involved in initial data analysis and drafting of the manuscript. Rajiv Gulati and Anjum Pervez had oversight of all the stages of the research and reviewed the final draft for academic content. Nazia Tauheed analysed the data and critically reviewed the final manuscript. All authors approved the final manuscript.