Intra-Abdominal Desmoid Tumour (DT) with Pelvic Extension-A Case Report
Sathish Selva Kumar1, Padmini Ramachandran2, Veena G3, Napa Madhusudhan4, Uday Kumbhar5
1 Assistant Professor, Department of Pathology, ESI Medical College and Post Graduate Institute of Medical Science and Research (PGIMSR),Tamil Nadu, India.
2 Senior Resident, Department of Pathology, ESI Medical College and Post Graduate Institute of Medical Science and Research (PGIMSR),Tamil Nadu, India.
3 Senior Resident, Department of Pathology, ESI Medical College and Post Graduate Institute of Medical Science and Research (PGIMSR),Tamil Nadu, India.
4 Assistant Professor, Department of Surgery, ESI Medical College and Post Graduate Institute of Medical Science and Research (PGIMSR),Tamil Nadu, India.
5 Associate Professor, Department of Surgery, ESI Medical College and Post Graduate Institute of Medical Science and Research (PGIMSR), K.K. Nagar, Chennai-600078, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sathish Selva Kumar, F-4, Shiv Apartments, No.1, 3rd Cross Street, Kalaimagalnagar, Ekkattuthangal, Chennai-600032, TamilNadu, India.
Phone: 09884858501,
E-mail: sathishpath17@gmail.com
Desmoid Tumour (DT) is a rare benign, myofibroblastic tumour originating from muscle fascia with tendency to recur but, it rarely metastasizes. We are reporting here a case of DT that presented as an intra-abdominal mass with pelvic extension in a patient who underwent hysterectomy for fibroid uterus seventeen years ago. A clinical diagnosis of ovarian malignancy was made. Ovarian tumour markers for surface epithelial and germ cell tumours were negative. Imaging studies suggested DT and the same was excised surgically. A histopathological diagnosis of DT was made and confirmed with immunohistochemistry (IHC) markers. DT should always be considered especially in female patients with previous history of surgery. A complete surgical excision is the treatment of choice with recurrent cases requiring radiotherapy. A differential diagnosis like sarcoma and further toxic chemotherapy can be avoided with careful histopathological evaluation and IHC confirmation of DTs.
Myofibroblast tumour, Desmoid tumour
Case Report
A female patient complained of vague pain since three months, with a past history of abdominal hysterectomy and right salphingo-oopherectomy, seventeen years ago for fibroid uterus.
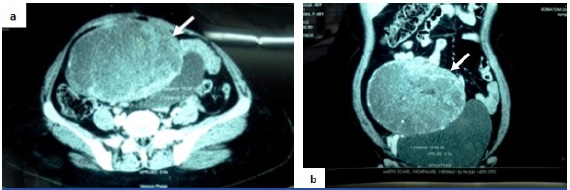
Examination and Investigation: On examination, a midline vertical scar in the abdomen due to previous surgery and a hard abdomino-pelvic mass of 14x11cm size reaching upto the umbilicus were noted. A clinical diagnosis of a right adnexal lesion probably an ovarian malignancy was made. Contrast Enhanced Computed Tomography (CECT) revealed a large, well defined, heterogeneously enhancing mass appeared to arise from the mesentery with transverse abdominis muscle abutment in the infra-umbilical region and predominantly situated in the lower abdomen extending upto the pelvis [Table/Fig-1]. It also caused mass effect over the bladder and the small bowel loops. The differential diagnoses of mesenteric desmoid, mesenteric Gastro Intestinal Stromal Tumour (GIST) and a right adnexal ovarian neoplasm were considered. Tumour markers for ovarian malignancy [Alpha Feto Protein (AFP), beta Human Chorionic Gonadotropin (HCG), Cancer Antigen 125 (CA 125)] were negative. Computed Tomography (CT) guided biopsy of the mass remained inconclusive. Intravenous Pyelogram (IVP) and colonoscopy were normal.
Contrast enhanced CT scan abdomen: a) axial section b) coronal section demonstrates a heterogeneously enhancing mass (white arrows) predominantly situated in the lower abdomen with pelvic extension causing mass effect over the bladder and small bowel loops
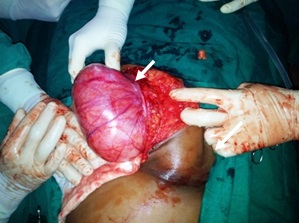
Exploratory laparotomy: Intra-operatively the tumour was attached to the posterior aspect of anterior abdominal wall, part of urachus, right side ureter and to the upper, right lateral part of the bladder wall [Table/Fig-2]. Right sided ureteric stenting was done prior to the exploration by the urologist. The attachments were released and the tumour was resected.
Intra-operative picture of the mass (white arrow)
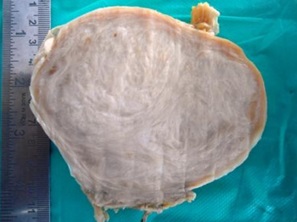
Histopathology: Gross examination showed a circumscribed mass, measuring 14x11x10cm with a smooth external surface. Cut section was glistening grey white with coarse trabeculations [Table/Fig-3].
Gross: Cut section reveals a grey white surface with coarse trabeculations
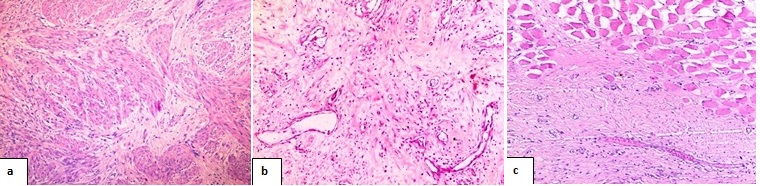
Microscopy: Microscopy revealed uniform spindled shaped cells, surrounded and separated from one another by abundant collagen with little cell to cell contact along with prominent dilated small vascular channels [Table/Fig-4]. The periphery showed entrapment of nearby muscle fibers. The nuclei were small, pale staining with one to three nucleoli and occasional mitoses. Resected margins were free of tumour.
Hematoxylin and eosin stained sections a) uniform spindled shaped cells, surrounded and separated from one another by abundant collagen (100x) b) prominent dilated small vascular channels are seen (100x) c) show entrapment of nearby muscle fibers (100x)
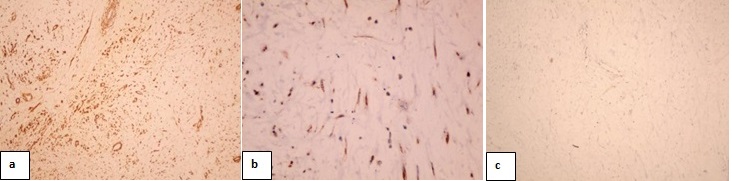
Immunohistochemistry (IHC): The mass was positive for smooth muscle actin, beta catenin, while negative for c-kit [Table/Fig-5]. A diagnosis of intra-abdominal DT with pelvic extension was made. Nine months on follow up, the patient was symptoms free.
Immunohistochemistry evaluation a) smooth muscle actin marker showed positive staining (100x) b) beta catenin marker showed focal positive staining (400x) c) c-kit marker showed negative staining (100x)
Discussion
Fibromatoses constitutes a benign group of myofibroblastic lesions with intermediate biologic behavior [1]. The World Health Organization (WHO) Committee for Classification of Soft Tissue Tumours in 2002 categorized them into superficial and deep, on the basis of anatomical locations [2]. Our case belongs to the deep fibromatoses group which occur in extra-abdominal areas (60%), abdominal wall (25%) and in intra-abdominal regions (15%) [3]. Intra-abdominal DTs include pelvic and mesenteric fibromatoses [1]. DTs have a female predilection of 87%, and 95% of them have already had atleast one childbirth [4]. No significant ethnic or racial predilection is noted [3].
DTs are predominantly idiopathic in origin and they can also be due to hormonal, traumatic and genetic factors [1]. Studies in guinea pigs have shown increased occurrence of tumours by prolonged estrogen administration and its prevention by testosterone, progesterone, and desoxycorticosterone administration [1]. Tumour regression after menopause and oophorectomy has been documented [5]. These findings suggested a role of the hormones in their development [1]. DTs occur in about 20% of cases after a surgical procedure while 50% of them occur in the first four years post procedure [5]. Cases occurring near a surgical scar are referred as “cicatrical fibromatoses” [6]. Molecular level interlinks between wound healing process and mesenchymal fibro-proliferative tumour development has been suggested [1]. In patients with no obvious trauma, minute muscle tears may contribute in susceptible individuals [1]. Its increased incidence in patients with familial adenomatous polyposis (FAP)/Gardner syndrome supports the genetic etiology [6]. These patients have a prevalence range from 3% to 34% with an increased risk of 1000 fold when compared with general population [7]. The FAP associated DTs are equally distributed among both the sexes with 40% being multiple and smaller [8]. Radiological investigations help in delineating the location, extent and adjacent structures involvement to assess local recurrence and help in further management [6].
The rarity of desmoid tumours cause difficulty in pre-operative diagnosis where only 50% of them are diagnosed correctly [9]. The intra-abdominal and pelvic fibromatoses can simulate an ovarian neoplasm in females, metastatic tumours, sarcoma, mesenteric GIST, sclerosing mesenteritis or inflammatory myofibroblastic tumour causing clinical dilemmas [1]. Grossly, DTs in this location are large and are dense, hard to rubbery in consistency, fairly circumscribed lesion with cut sections showing trabeculations [1]. Microscopically they are dense collagenous lesions with spindle cells forming intertwining sweeping bundles. They consist of uniform cells lacking mitotic activity with no pleomorphism or necrosis. The periphery of the tumour often reveals entrapped muscle fibers. Mucinous areas and dilated blood vessels are usually seen in deep fibromatoses [1]. In fibrosarcoma the lesion is more cellular, separated by less collagen, a more consistent sweeping fascicular (herringbone) pattern, hyperchromatic, atypical nuclei and high mitoses (>1/10 high power fields) [1]. Sclerosing mesenteritis and inflammatory myofibroblastic tumour have variable amount of fibrosis and inflammation [1]. DTs are positive for smooth muscle actin, beta catenin, vimentin, muscle specific actin and occasionally positive for desmin markers. Somatic beta catenin or adenomatous polyposis coli gene mutations cause intranuclear accumulation of beta catenin. A mesenteric GIST can be excluded by histological criteria, beta catenin positivity and c-kit negativity [1].
DTs usually arise from musculo-aponeurotic structures of rectus abdominis, internal oblique, transverse abdominis muscles [1]. The present case possibly had arisen from the posterior aspect of the transverse abdominis muscle fascia with intra-abdominal and pelvic extension. They are usually solitary, slow growing, therefore large at presentation with infiltrative pattern of growth and frequent recurrences causing varied clinical presentations [3].
Majority of them are managed with wide local surgical resection [6]. Abdominal wall reconstruction may be necessary via prosthetic fascial augmentation and local fasciocutaneous advancement or regional flaps [10]. High recurrence rates can be managed with adjuvant and/or neo-adjuvant radiotherapy [1]. Other modalities such as radio frequency ablation, chemical ablation and gene transfer methods are in their early stages [11].
Conclusion
DTs are rare. The diagnostic dilemma is due to its prominent discrepancy between the bland microscopic appearance and its tendency for recurrence. DT should always be a differential entity especially in females with previous history of surgery. To differentiate from sarcoma and thereby avoiding toxic chemotherapy effects, a complete clinical, radiological, biochemical and histopathological evaluation becomes essential. Multi-specialty expertise is often required in the diagnosis and treatment of deep intra-abdominal and pelvic DTs.
[1]. Weiss SW, Goldblum JR, Soft tissue tumours: Fibromatoses 2008 Fifth editionPhiladelphiaMosby Elsevier:227-56. [Google Scholar]
[2]. Fletcher CD, Unni KK, Mertens F, World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone 2002 LyonIARC Press [Google Scholar]
[3]. Gupta Shahana, Ray Udipta, Chatterjee Souvik, Kumar S, Satapathy A, Chatterjee S, Sporadic intra-abdominal desmoid: a rare presentation as a hepatic massCase Reports in Pathology 2012 Article ID 245671, 5 pages [Google Scholar]
[4]. Miettinen MM, Diagnostic soft tissue pathology 2003 New York, NYChurchill Livingstone [Google Scholar]
[5]. Siegelman ES, Body MRI 2005 Philadelphia, PaElsevier [Google Scholar]
[6]. Murphey MD, Ruble CM, Tyszko SM, Zbojniewicz AM, Potter BK, Miettinen M, RadioGraphics 2009 29:2143-76. [Google Scholar]
[7]. Kobayashi H, Kotoura Y, Hosono M, MRI and scintigraphic features of extraabdominal desmoid tumoursClin Imaging 1997 21:35-39. [Google Scholar]
[8]. Clark SK, Neale KF, Landgrebe JC, Phillips RK, Desmoid tumours complicating familial adenomatous polyposisBr J Surg 1999 86:1185-89. [Google Scholar]
[9]. Lopez R, Kemalyan N, Moseley HS, Dennis D, Vetto RM, Problems in diagnosis and management of desmoid tumoursAm J Surg 1990 159:450-53. [Google Scholar]
[10]. Sorensen A, Keller J, Nielsen OS, Jensen OM, Treatment of aggressive fibromatosis: a retrospective study of 72 patients followed for 1–27 yearsActa Orthop Scand 2002 73:213-19. [Google Scholar]
[11]. Dozois EJ, Desmoid diseaseMayo Clinic gastrointestinal Surgery 2004 PhiladpelphiaSaunders:563-65. [Google Scholar]