Leukostasis in an Adult with AML Presenting as Multiple High Attenuation Brain Masses on CT
Abdulaziz Ahmad Algharras1, Alexander Mamourian2, Thomas Coyne3, Suyash Mohan4
1 Faculty, Department of Radiology, Qassim University, Saudi Arabia.
2 Professor, Department of Radiology, Hospital of the University of Pennsylvania, USA.
3 Forensic Pathology Fellow, Department of Miami-Dade Medical Examiner, Number Oneon Bob Hope Rd., Miami, Fl 33136.
4 Assistant Professor, Department of Radiology, Hospital of the University of Pennsylvania, USA.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Alexander Mamourian, 219 Dulles, 3400 Spruce St., Philadelphia, PA 19104.
Phone: 215 662 6865, Fax: 215 662 3283,
E-mail: Alexander Alexander.Mamourian@uphs.upenn.edu
Acute myeloid leukemia (AML) is a hematologic malignancy that can present with central nervous system (CNS) symptoms. Neurological symptoms may result from the local accumulation of malignant cells in or near the brain (chloroma), infection, hemorrhage, or infarcts from leukostasis. Leukostasisis a syndrome that can include brain infarction due hyperviscosity of blood with vascular occlusion but CNS involvement is rarely encountered in adults. We report an unusual case of leukostasis in an adult who presented with multiple high attenuation intracranial masses on CT. While initially thought to represent chloromasthey proved to be hemorrhagic infarcts secondary toleukostasis on open brain biopsy. This condition is under-reported in the radiology literature and only rarely biopsy proven. We review in this paper the pathological, CT and MRI findings of leukostasis in order to increase awareness of this uncommon entity and facilitate diagnosis.
Leukostasis, Chloroma, AML
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy with presenting symptoms ranging from those of bone marrow failure, such as anaemia, neutropenia, and thrombocytopenia, or resulting from organ infiltration with leukemic cells, or both. Neurological symptoms may result from the local accumulation of malignant cells in or near the brain (chloroma), infection, haemorrhage, or infarcts from leukostasis [1]. Leukostasis is a clinico-pathologic syndrome that results in intravascular thrombosis due to elevated blood viscosity from the very high white blood cell count, with subsequent brain infarction [2].
We report an unusual case of leukostasis in an adult patient with a known diagnosis of AML who presented with neurological symptoms and multiple high attenuation intracranial masses on CT. These were initially thought to represent chloromas on the basis of head CT appearance, but subsequently proved to be hemorrhagic infarcts secondary to leukostasis on open brain biopsy. This condition is under-recognised in the radiology literature, unusual in adults and even rarer to be biopsy proven. Our intent with this paper is to review the pathological findings in leukostasis and illustrate the relevant imaging features of this uncommon disease process.
Case Report
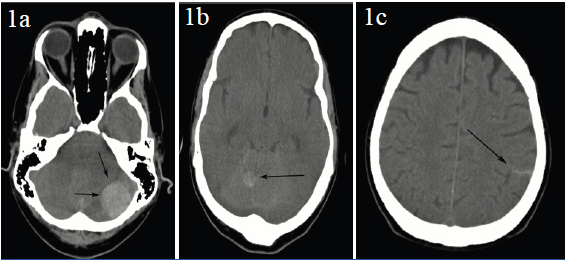
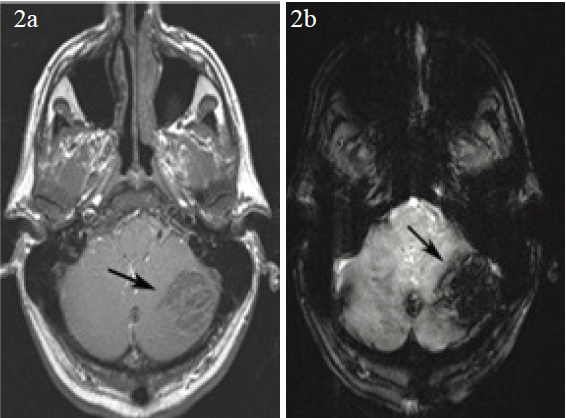
A 51-year old male with a history of diffuse large B cell lymphoma, status post bone marrow transplant was in remission for three years, when he developed AML. He then presented to the emergency room with recurrent fevers, temperature to 101° F, but without other systemic symptoms. He was managed conservatively but he then began to experience headaches associated with nausea and vomiting. There was history of minor head trauma prior to these new symptoms. A head CT was obtained that revealed multiple high attenuation intracranial masses along with subarachnoid haemorrhage [Table/Fig-1]. MRI of the brain was performed for further characterisation of these hyperdense masses, which revealed similar findings with additional smaller, lesions in multiple compartments [Table/Fig-2]. Based on the CT chloromas were considered in view of patients known AML, but the presence of associated subarachnoid haemorrhage was atypical. Multiple traumatic haemorrhages was another consideration, since the patient was chronically thrombocytopenic, platelet count <10,000 and WBC count of 22,000, but the distribution of these high attenuation lesions was not at all typical for traumatic haemorrhages either. Hemorrhagic leukemic infiltrates remained a consideration in the differential.
Axial CT scan (1a) demonstrates one of many high attenuation masses, this one in the left cerebellar hemisphere (arrows). A second lesion is evident in the right cerebellar hemisphere (1b, arrow) as well as supratentorial subarachnoid haemorrhage (1c, arrow)
Axial post contrast enhanced MRI (2a) shows no evidence of enhancement in the left hemispheric lesion (arrow). The shallow flip angle gradient echo scan (2b) shows marked susceptibility effect from haemorrhage in the left cerebellar lesion (arrow). The presence of haemorrhage would explain its high attenuation on CT
Radiation-oncology was consulted for consideration of treatment of the brain lesions but a tissue diagnosis became necessary because of the unusual imaging features of these lesions. Neurosurgery performed a craniotomy in order to provide a tissue sample as well as provide decompression of the posterior fossa.
Histology Findings
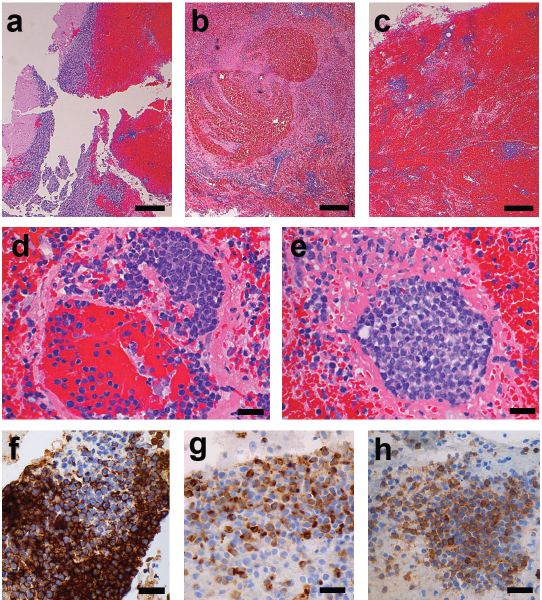
Sections from the tissue sample showed fragments of cerebellum displaced by organising haemorrhage. Within the hemorrhagic brain parenchyma there were scattered mononuclear cell infiltrates in an angiocentric pattern [Table/Fig-3]. Numerous thrombosed vessels were identified containing myeloblastic cells admixed with fibrin forming leukostatic tumor thrombi. Scattered necrotic vessels were observed with variable fibrinoid change and accompanying transmural infiltration of myeloblastic cells and perivascular cuffing. Multiple proliferative myeloblasts were present within vessel lumens and within congested perivascular spaces. Immunohistochemical stains were consistent with acute myelogenous leukemia. Overall the histology was characteristic of acute hemorrhagic infarction secondary to blastic leukostasis. Similar pathology finding has been discussed in Koenig MK and others [3].
(a) Fragments of cerebellar parenchyma (left) effaced by haemorrhage. (b,c) Low power micrograph demonstrates organizing haemorrhage containing multiple small vessels cuffed by proliferating myeloblasts. (d) Infarcted vessel containing thrombus comprised of fibrin and intraluminal blasts with extension into the perivascular space. (e) Infarcted vessel completely occluded by leukostatic myeloblasts. (f-h) Immunohistochemical stains highlight CD43 (f), myeloperoxidase (g), and ckit (h) immunoreactivity within the leukostatic myeloblasts (Scale bars: a,b=200 μm; c-h=100 μm)
Discussion
The differential diagnosis for any intracranial mass in a patient with AML includes three differential conditions: haemorrhage, chloroma, and infection. A chloroma represents a localised myeloblastic tumor that is also called a granulocytic sarcoma. It can involve brain, bone, periosteum, lymph nodes, and skin. It has been described in patients with other myeloproliferative and myelodysplastic disorders but these lesions are most often encountered in patients with myeloblastic leukemias, especially AML [4]. Intracranial chloromas are believed to represent leukemic cell collections that pass directly from the calvarial bone marrow to the dura, occasionally with invasion of the subarachnoid space and parenchyma via the Virchow–Robin spaces.
Cloromas on CT usually appear as a homogenous, high attenuation masses with homogeneous enhancement after administration of intravenous contrast. Their high attenuation is most likely due to dense cell packing and they are not reported to haemorrhage. Because of their typical extra-axial location chloromas can be easily be mistaken for a meningioma, particularly if the clinical context is unknown to the imager [5]. On MRI, chloromas appear isointense to gray matter on T1-weighted images and show homogeneous enhancement after administration of intravenous gadolinium. While they are most often found in the extra-axial space, true intraparenchymal chloromas may be rarely encountered. In our case, presence of haemorrhage and lack of enhancement were atypical for chloromas.
Leukostasis, or symptomatic hyperleukocytosis, is an oncologic emergency, and may be diagnosed clinically when the white blood cell count (WBC) is over 100 x 109/L (100,000/μL) in patients with leukemia. WBCs’ are much less flexible than red blood cells, but since the number of circulating WBCs is so small in healthy patients, there is a negligible effect on overall blood viscosity. In patients with hematologic malignancies, the extremely high number of WBCs can result in hyperviscosity, as these cells are not easily deformable and accumulate in capillaries. Malignant WBCs are even less malleable than normal WBCs (with malignant myeloid cells more viscous than malignant lymphocytes). Additionally, leukemic cells may also promote formation of high number of adhesion factors, resulting in substantial attachment of leukemic cells to capillary endothelium— increasing chances of clinically apparent leukostasis. These patients may present with symptoms of tissue hypoxia, most commonly in the brain or lungs. Occasionally, as in this case, patients may have pathologically proven leukostasis at WBC counts below 100,000/μL.
Microscopic intravascular leukemic nodules have been reported in patients in blast crisis but unlike chloromas, which are solid tumors, these much smaller nodules are the result of intravascular aggregation of blast cells and are the cause of leukostasis [5]. These lesions are usually found in the deep white matter and are believed to be the result of stasis due to the intravascular aggregation of blast cells with resulting nodule formation. Their formation can furthermore lead to vessel wall disruption and subsequent haemorrhage [6].
The diagnosis of leukostasis is established when a tissue biopsy demonstrates leukostatic plugs in the microvasculature, as seen in our case. However, pathologic diagnosis of cerebral leukostasis is rarely available due to the risks associated with brain biopsy [5]. In our case the patient’s white cell count was lower that expected for this diagnosis, 47,500/uL was the highest count pre-operatively, which contributed to the decision to obtain a brain biopsy.
Conclusion
In adult patients with AML with high attenuation masses on head CT, while chloroma is more common, consideration should be given to hemorrhagic infarcts due to leukostasis even if the patient has only moderate elevation of WBC. MR imaging with contrast should be of value in establishing the correct diagnosis since homogeneous enhancement, typical of chloromas, will be absent in cases of leukostasis.
[1]. Sullivan MP, Intracranial complications of leukemia in childrenPediatrics 1957 20:757-81. [Google Scholar]
[2]. Ginsberg LE, Leeds NE, Neuroradiology of LeukemiaAm J Roentgenol 1995 165:525-34. [Google Scholar]
[3]. Koenig MK, Sitton CW, Wang M, Slopis JM, Central nervous system complications of blastic hyperleukocytosis in childhood acute lymphoblastic leukemia: diagnostic and prognostic implicationsJ Child Neurol 2008 23:1347-52. [Google Scholar]
[4]. Muss HB, Moloney WC, Chloroma and other myeloblastic tumorsJ Blood 1973 42:721-28. [Google Scholar]
[5]. Leonard KJ, Mamourian AC, MR Appearance of intracranial chloromasAm J Neuroradiol 1989 10:S67-68. [Google Scholar]
[6]. Freireich EJ, Thomas LB, Frei E, Fritz RD, Forkner CE, A distinctive type of intracerebral haemorrhage associated with “blastic crisis” in patients with leukemiaCancer 1960 13:146-54. [Google Scholar]