Immunohistochemical Detection of p16INK4a in Leukoplakia and Oral Squamous Cell Carcinoma
Pradyot Prakash1, Muktesh Khandare2, Mohan Kumar3, Rahul Khanna4, Gyan Prakash Singh5, Gopal Nath6, Anil Kumar Gulati7
1 Assistant Professor, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India.
2 Senior Resident, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India.
3 Professor, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India.
4 Professor, Department of General Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India.
5 Associate Professor, Department of Community Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India.
6 Professor, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India.
7 Professor, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Pradyot Prakash, Assistant Professor, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India.
Phone: +91 9935345667,
E-mail: pradyotbhu@gmail.com
Introduction: Over-expression of p16INK4a has been reported in tissues of oral squamous cell carcinoma (SCC) associated with Human Papillomaviruses (HPVs). Immunohistochemical (IHC) detection of p16INK4a is an easy technique than molecular detection of HPVs, hence we investigated the presence of this protein in the most common pre-malignant and malignant oral lesions i.e. leukoplakia and SCC respectively.
Material and Methods: We performed IHC detection of p16INK4a in sections of paraffin embedded formalin fixed tissues of leukoplakia with or without dysplasia (n= 21) and SCC lesions (n= 69) and correlated with various patterns of p16INK4a positivity with respect to histological diagnosis.
Results: In the present study, 71% cases of oral SCC cases were positive for p16INK4a, of which the most common pattern was diffuse nuclear and cytoplasmic staining. Among the cases with leukoplakia, 57.1% were positive for overexpression of p16INK4a, wherein diffuse and sporadic pattern was observed among 23.8 percent each.
Conclusion: In the present study, significant number of oral SCC cases observed overexpressing p16INK4a . However HPV DNA detection based studies are needed to validate the utility of IHC detection of p16INK4a as a surrogate marker for HPV associated oral SCC.
p16, Oral carcinoma, leukoplakia, HPV
Introduction
Oral carcinoma is amongst the leading malignancies worldwide, with an overall incidence of 16.1 adults per 100,000, with marked geographic variation in its distribution [1]. It ranks number one among all cancers in males and third in females in India [2]. Squamous cell carcinoma (SCC) is the commonest of all oral malignancies. It has been observed that 5.7% of leukoplakia, the most common oral pre malignant mucosal lesion, may get transformed into malignant lesion every year [3].
The aetiology of oral carcinoma has been considered to be multifactorial. Several epidemiological data suggests a strong association between oral cancer and risk factors like cigarette smoking, smokeless tobacco and alcohol consumption [4–6]. Further, there is an ample evidence of association between chewing betel quid with and without tobacco and oral squamous cell carcinoma [7]. Recently, certain high Risk Human Papillomaviruses (HPV) genotypes were found associated with potentially pre-malignant and malignant oral lesions [8].
One of the several cyclin-dependent kinase inhibitors, which are responsible for regulation of normal cell cycle, p16INK4a is usually inactivated in many cancers through mutation, deletion or hypermethylation of the gene, resulting in reduced or loss of expression. But in situation of cellular transformation, in which pRB is directly inactivated by E7 oncogene of some of the high risk HPVs, cells are released from growth-suppressive stimuli mediated by the p16INK4a. This leads to the conclusion that reduced or lost pRB function results in enhanced p16INK4a levels, as a result of a negative feedback control [9].
Expression of p16INK4a in association with HPV-HR infection has been observed in a high proportion of cases with high grade cervical dysplasia and cancer. Recently, it has been observed that those cases of oropharyngeal carcinoma, which are associated with transcriptionally active HPV DNA may need deintensified regimens which will reduce the long term negative impact of treatment. Such cases may be singled out by IHC detection of p16INK4a [10,11].
In the present study, we have investigated p16INK4a expression in oral pre-malignant lesion i.e. leukoplakia and oral SCC; and to correlate patterns of p16INK4a positivity with respect to different histological grades of oral SCC.
Material and Methods
On the basis of clinical features and histopathological confirmation 21 patients of leukoplakia and 69 patients of SCC were included in the present study. These cases presented in surgical OPD of the University Hospital of Banaras Hindu University, Varanasi, India. between January 2011 and June 2012. The age of the patients with leukoplakia ranges from 16 and 75 years and that of SCC was between 22 and 70 years. Punch biopsy samples were taken from each patient and subjected to haematoxylin and eosin staining as per standard protocol for histopathological confirmation and grading of the lesions [12].
The IHC detection of p16INK4a expression was performed on tissue sections, prepared from paraffin embedded formalin fixed tissues, by using p16INK4a monoclonal antibody kit (BioGenex). Positive controls included block sections of HeLa cell line (HPV 18 transfected). Primary antibody was replaced with PBS in negative control and normal oral tissue in each assay. Immunostaining of the sections was reviewed and a strong nuclear as well as cytoplasmic staining was considered as positive reaction, as described by Klaes et al., [9]. The distribution of p16INK4a positivity was scored as negative (<1% cells positive), sporadic (<5% cells positive), focal (<25% cells positive) and diffuse (>25% cells positive).
The x2 test was applied to calculate the significance of association of p16INK4a overexpression with oral pre-malignant and malignant lesions. The study was duly approved by the Institute Ethics Committee of Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
Results
In the present study, cases of leukoplakia were observed in all age group; however 81% of these were seen above 30 years of age. Among oral SCC group, 94.2% of the patients were observed above 30 years, however none of the cases were observed below 20 years of age [Table/Fig-1]. Further, observing the sex distribution among the subjects, premalignant and malignant lesions was observed more among males constituting 80.9% and 84.1% respectively [Table/Fig-2].
Age distribution of subjects of Leukoplakia and Oral SCC
| Age group | Leukoplakia (n=21 ) | Oral SCC (n=69 ) |
|---|
| <20 | 2 (9.5) | – |
| 21-30 | 2 (9.5) | 4 (5.8) |
| 31-40 | 6 (28.6) | 10 (14.5) |
| 41—50 | 3 (14.3) | 20 (29.0) |
| 51-60 | 6 (28.6) | 19 (27.5) |
| ≥ 61 | 2 (9.5) | 16 (23.2) |
Note: Data in parenthesis indicate percentage.
Sex distribution of subjects of Leukoplakia and Oral SCC
| Leukoplakia ( n=21) | Oral carcinoma (n=69) |
|---|
| Male | Female | Male | Female |
|---|
| 17 (80.9) | 4 (19.1) | 58 (84.1) | 11 (15.9) |
Note: Data in parenthesis indicate percentage
Tongue was the most common site involved in SCC followed by buccal mucosa, cheek, alveolus, soft palate, lip, angle of mouth, hard palate and gingiva. Among oral pre malignant lesions most common sites involved were tongue and buccal mucosa followed by cheek, lip, alveolus and angle of mouth and hard palate [Table/Fig-3]. Majority of oral SCC cases, in our study, were of grade-1 (53/69, 76.8%), followed by grade-2 13/69, 18.8%) and grade-3 (3/69, 4.4%) on histopathological grading after H & E staining. Out of total of 21 pre malignant lesions 7 cases (33.33%) were of leukoplakia with dysplasia and 14 (66.67%) were without dysplasia.
Distribution of sites of Leukoplakia and Oral SCC
| Sites of the Lesions | Leukoplakia (n=21) | Oral SCC (n=69) |
|---|
| Tongue | 5 (23.8) | 26 (37.7) |
| Buccal mucosa | 5 (23.8) | 17 (24.6) |
| Cheek | 4 (19) | 13 (18.8) |
| Alveolus | 2 (9.5) | 4 (5.8) |
| Lip | 3 (14.2) | 2 (2.9) |
| Angle of mouth | 1 (4.8) | 2 (2.9) |
| Soft palate | – | 3 (4.3) |
| Hard palate | 1 (4.8) | 1 (1.45) |
| Gingiva | – | 1 (1.45) |
Note: Data in parenthesis indicate percentage
While observing for overexpression of p16INK4a by IHC, 71.01% cases of oral carcinoma were found to be positive, whereas in oral leukoplakic lesions the positivity was 57.14%. While observing the overexpression in oral cancers, out of 69 cases 31.9% had diffuse pattern [Table/Fig-4] followed by sporadic and focal [Table/Fig-5] which were observed in 24.6 and 14.5% cases respectively. Among 21 leukoplakia cases, 23.8% cases were found to exhibit diffuse and sporadic patterns of p16INK4a expression each and focal expression was observed in 9.5% cases [Table/Fig-6].
IHC detection of diffuse expression of p16 INK4a in SCC (400x magnification)
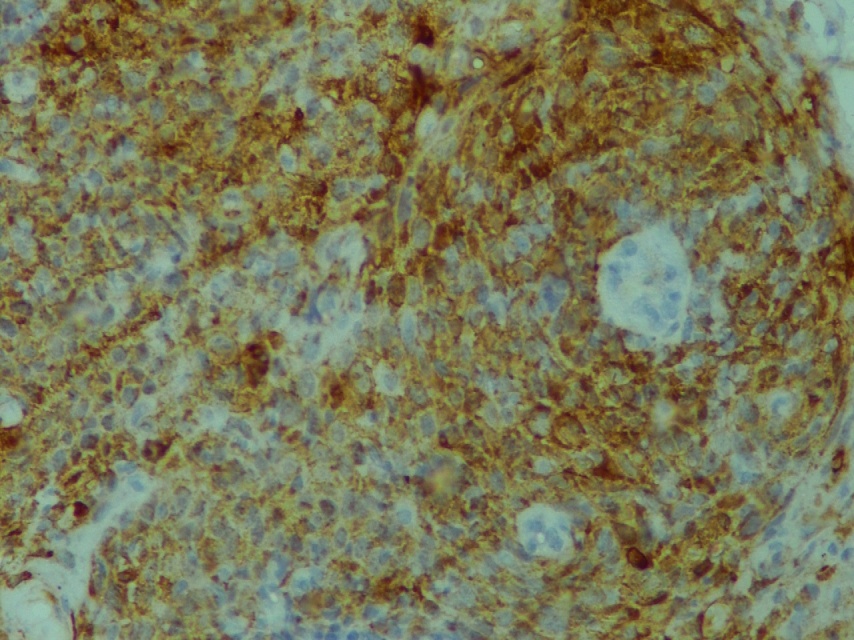
IHC detection of focal expression of p16 INK4a in SCC (400x magnification)

p16INK4a expression in Leukoplakia and Oral SCC
| Lesions | Negative | Sporadic | Focal | Diffuse | Total |
|---|
| n | % | n | % | n | % | n | % |
|---|
| Leukoplakia | 9 | 42.8 | 5 | 23.8 | 2 | 9.6 | 5 | 23.8 | 21 |
| OSCC | 20 | 29.0 | 17 | 24.6 | 10 | 14.5 | 22 | 31.9 | 69 |
| 29 | 32.2 | 22 | 24.5 | 12 | 13.3 | 27 | 30 | 90 |
*OSCC = Oral Squamous cell carcinoma, = 24.37, P-value <0.05.
Among cases of leukoplakia without dysplasia, majority (38.5%) exhibited sporadic pattern of p16INK4a expression whereas most common pattern of overexpression among leukoplakia with dysplasia was diffuse (25%). Further, out of 69 cases of SCC, diffuse pattern of p16INK4a expression was observed in 28.3%, 42.9% and 50% cases of SCC grade 1, 2 and 3 respectively whereas focal staining among 15.1, 7.1 and 50 percent respectively. Further, none of the SCC grade 3 cases exhibited sporadic pattern of expression of p16INK4a but this pattern was observed 28.3% and 14.3% cases among SCC grade 1, and 2 respectively [Table/Fig-7].
p16INK4a expression in different grade in Leukoplakia and Oral SCC
| Lesions | Negative | Sporadic | Focal | Diffuse | Total |
|---|
| n | % | n | % | n | % | n | % |
|---|
| Leukoplakia without dysplasia | 4 | 30.7 | 5 | 38.5 | 1 | 7.7 | 3 | 23.1 | 13 |
| Leukoplakia with dysplasia | 5 | 62.5 | 0 | 0 | 1 | 12.5 | 2 | 25 | 8 |
| OSCC Grade 1 | 15 | 28.3 | 15 | 28.3 | 8 | 15.1 | 15 | 28.3 | 53 |
| OSCC Grade 2 | 5 | 35.7 | 2 | 14.3 | 1 | 7.1 | 6 | 42.9 | 14 |
| OSCC Grade 3 | 0 | 0 | 0 | 0 | 1 | 50 | 1 | 50 | 2 |
| Total | 29 | 32.2 | 22 | 24.4 | 12 | 13.3 | 27 | 30.1 | 90 |
*OSCC = Oral Squamous cell carcinoma
Discussion
Oral cancers continues to be a public health problem with an estimated incidence of 267,000 cases and 128,000 deaths annually, two-thirds of which is observed in developing countries [13]. Recently, it has been observed that age standardized incidence rate of oral cancer per 100 000 population is 12.6 in India [14].
In the present study, tongue was observed to be the commonest site affected by SCC, while leukoplakia was equally observed on tongue and buccal mucosa. Hard palate was observed to be the least affected by such lesions. Although, tongue was not the preferred site for development of leukoplakia, but this was one of the most probable site to show dysplastic or malignant changes [15].
The reports implicating specific HPV types in oral carcinoma were first published in 1985 [16,17]. It has been observed that HPV 16 participates in disruption of regulation of p16INK4a suppressor protein and its overexpression can be used as surrogate marker for detection of HPV 16 association in oral SCC [18,19]. Similarly, the value of the immunostain for p16INK4a was observed in identifying dysplasic lesions [20]. It was observed that there are two subsets of oral SCC and only one subset is associated with HPV infection, with two different mechanisms working at genetic level [21]. The present study also indicates two different subsets of these lesions on the basis of p16INK4a expression as 71.01% cases of oral SCC, and 57% cases of leukoplakia were positive for the overexpression of the said protein.
Further, It has been observed that p16INK4a expression is strong independent prognostic indicator also. The patients with oral SCC, not expressing p16INK4a had 4 times increase risk of death and 7.5 times increase risk of recurring cancer in comparison to those expressing it. Prognosis of p16INK4a positive cases has been reported to be better irrespective of histological grade [22].
On statistical analysis, p16INK4a over expression (sporadic, focal and diffuse pattern combind) in cases of pre malignant lesions observed to be insignificant (p> 0.05) but was found to be strongly associated with oral SCC (p< 0.05). According to a recently published meta-analysis, p16INK4a positivity range from 12.8 to 100% in oral carcinoma cases, which might be due to use of different kits for IHC detection of p16INK4a by different groups of investigators [23].
While observing p16INK4a positivity in leukoplakia with or without dysplasia as well as different grades of SCC in this study, no single pattern of expression alone was strongly associated with leukoplakia or SCC cases. However, a study conducted earlier observed focal and diffuse pattern in HPV positive cases, of both premalignant as well as SCC cases, in a statistically significant number of cases [24]. On the contrary, it has been reported that SCC of cervix, caused by certain high risk genotypes of HPV, exhibits significant association with diffuse pattern of expression of p16INK4a [25]. As we did not attempt for HPV DNA detection it would not be imperative for us to comment on the specificity of p16INK4a expression regarding association of HPV in oral SCC.
Conclusion
The present study demonstrated the significant association of p16INK4a overexpression in cases of oral SCC. Further HPV DNA detection based studies are needed to validate the utility of IHC detection of p16INK4a as a surrogate marker for HPV associated oral SCC.
Note: Data in parenthesis indicate percentage.
Note: Data in parenthesis indicate percentage
Note: Data in parenthesis indicate percentage
*OSCC = Oral Squamous cell carcinoma, = 24.37, P-value <0.05.
*OSCC = Oral Squamous cell carcinoma
[1]. National Cancer Institute [Internet]. Surveillance Epidemiology and End Results: SEER Stat Fact Sheets: Oral Cavity and Pharynx - [cited 2013 Feb 22]. Available from: http://www.seer.cancer.gov/statfacts/html/oralcav.html#incidence-mortality [Google Scholar]
[2]. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer 2010 127:2893-917. [Google Scholar]
[3]. Lee JJ, Hong WK, Hittelman WN, Mao L, Lotan R, Shin DM, Predicting cancer development in oral leukoplakia: ten years of translational researchClin Cancer Res 2000 6:1702-10. [Google Scholar]
[4]. IARC Monographs on the evaluation of carcinogenetic risk to humans tobacco smokingLyon 2002 vol. 83 [Google Scholar]
[5]. IARC Monographs on the evaluation of carcinogenetic risk to Smokeless Tobacco and Some Tobacco-specific N-NitrosaminesLyon 2007 vol. 89 [Google Scholar]
[6]. IARC Monographs on the evaluation of carcinogenetic risk to humans. Alcohol Consumption and Ethyl CarbamateLyon 2010 vol. 96 [Google Scholar]
[7]. IARC Monographs on the evaluation of Betel-quid and Areca-nut Chewing and Some Areca-nut-derived NitrosaminesLyon 2004 vol. 85 [Google Scholar]
[8]. Bouda M, Gorgoulis VG, Kastrinakis NG, Giannoudis A, Tsoli E, Danassi-Afentaki D, “High risk” HPV types are frequently detected in potentially malignant
and malignant oral lesions, but not in normal oral mucosaMod Pathol 2000 13:644-53. [Google Scholar]
[9]. Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Overexpression of p16INK4A as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteriInt J Cancer 2001 92:276-84. [Google Scholar]
[10]. Tribius S, Ihloff AS, Rieckmann T, Petersen C, Hoffmann M, Impact of HPV status on treatment of squamous cell cancer of the oropharynx: what we know and what we need to knowCancer Lett 2011 304:71-9. [Google Scholar]
[11]. Lewis JS Jr, p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinomaHead Neck Pathol 2012 6:75-82. [Google Scholar]
[12]. Gamble M, Hematoxylins and Eosin. In: Bancroft J.D., Gamble M, editorsTheory and Practice of Histological Technique 2008 6th edEndinburghChurchill Livingstone [Google Scholar]
[13]. WHO Certified [Internet]. IARC Screeing group. Oral cancer - [cited 2013 Feb 22]. Available from: http://screening.iarc.fr/oralindex.php [Google Scholar]
[14]. Petersen PE, Oral cancer prevention and control--the approach of the World Health OrganizationOral Oncol 2009 45:454-60. [Google Scholar]
[15]. Neville BW, Day TA, Oral cancer and precancerous lesionsCA Cancer J Clin 2002 52:195-215. [Google Scholar]
[16]. Löning T, Ikenberg H, Becker J, Gissmann L, Hoepfer I, zur Hausen H, Analysis of oral papillomas, leukoplakias, and invasive carcinomas for human papillomavirus type related DNAJ Invest Dermatol 1985 84:417-20. [Google Scholar]
[17]. de Villiers EM, Weidauer H, Otto H, zur Hausen H, Papillomavirus DNA in human tongue carcinomasInt J Cancer 1985 36:575-78. [Google Scholar]
[18]. Klussmann JP, Gültekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, Eckel HE, Pfister HJ, Fuchs PG, Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirusAm J Pathol 2003 Mar 162(3):747-53. [Google Scholar]
[19]. Agrawal GP, Joshi PS, Agrawal A, “Role of HPV-16 in Pathogenesis of Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma and Correlation of p16INK4A Expression in HPV-16 Positive Cases: An Immunohistochemical Study.”ISRN Pathology 2013 (2013) [Google Scholar]
[20]. Dragomir LP, Simionescu C, Mrgritescu C, Stepan A, Dragomir IM, Popescu MR, P53, p16 and Ki67 immunoexpression in oral squamous carcinomasRom J Morphol Embryol 2012 53(1):89-93. [Google Scholar]
[21]. Chen SF, Yu FS, Chang YC, Fu E, Nieh S, Lin YS, Role of human papillomavirus infection in carcinogenesis of oral squamous cell carcinoma with evidences of prognostic associationJ Oral Pathol Med 2012 Jan 41(1):9-15. [Google Scholar]
[22]. Reimers N, Kasper HU, Weissenborn SJ, Stützer H, Preuss SF, Hoffmann TK, Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancerInt J Cancer 2007 12:1731-8. [Google Scholar]
[23]. Pérez-Sayáns M, Suárez-Peñaranda JM, Gayoso-Diz P, Barros-Angueira F, Gándara-Rey JM, García-García A, p16(INK4a)/CDKN2 expression and its relationship with oral squamous cell carcinoma is our current knowledge enough?Cancer Lett 2011 306:134-41. [Google Scholar]
[24]. Fregonesi PA, Teresa DB, Duarte RA, Neto CB, de Oliveira MR, Soares CP, p16(INK4A) immunohistochemical overexpression in premalignant and malignant oral lesions infected with human papillomavirusJ Histochem Cytochem 2003 51:1291-97. [Google Scholar]
[25]. Gupta R, Srinivasan R, Nijhawan R, Suri V, Uppal R, Protein p 16INK4A expression in cervical intraepithelial neoplasia and invasive squamous cell carcinoma of uterine cervixIndian J Pathol Microbiol 2010 Jan-Mar 53(1):7-11. [Google Scholar]