Halitosis, or oral malodor, is a common complaint of upto one-third of the general population and a large concern for any individual whom it affects [1,2]. It has been recently hypothesised that the tongue dorsum may be an important area of microbial metabolism causing putrefaction, leading to halitosis [3]. This is due to the fact that the tongue has a large surface area supporting a high number of oral bacteria that are exposed to nutrient sources.
Tongue coating is an important factor in the formation of oral malodor in both periodontally diseased and healthy people [3]. However, it is not known which bacterial species in the tongue coating are responsible for this odor production. It is possible that malodorous species colonising the dorsal surface of the tongue are the same as those found in subgingival plaques. Indeed studies suggest that the flora on the tongue is similar to odor-producing periodontal bacteria [4,5]. However, these studies examined the flora present in individuals who had no complaints of malodor; no one has simultaneously monitored the bacteriological flora of the tongue, plaque samples and malodor levels in patients with subjective complaints of halitosis. On the other hand, tongue coating is believed to be the main source of volatile sulphur compounds (VSC) production in orally healthy subjects [6,7]. More than 100 bacteria may be attached to a single epithelial cell desquamated from the tongue dorsum, whereas only about 25 bacteria are attached to each cell in other areas of the oral mucosa [8]. Another study has described the volume of tongue coating in subjects with oral malodor as significantly higher than in controls [7]. It is known that removal of tongue coating reduces VSC production in mouth air from orally healthy subjects without periodontal or gingival disease [9].
Common methods for the detection or determination of these oral malodor producing bacteria include cultivation, immunological procedures, benzoyl –DL- arginine-naphthylamide (BANA) test and the detection of typical metabolites. Furthermore, nucleic acid based procedures such as hybridisation techniques and Polymerase chain reaction (PCR) have become increasingly important. Among them, PCR offers the lowest limit of detection for bacteria (fewer than 10 cells per sample) [10]. Polymerase chain reaction based diagnostic technique has become a powerful and increasingly popular tool due to its rapidity, sensitivity and specificity [11].
Information regarding tongue coating & cleaning dates back to earlier century, however, this concept is ignored. The importance of tongue cleaning in oral malodor is revoked recently. Medline search using key words halitosis; chronic periodontitis; organoleptic; portable; tongue; microbes; polymerase chain reaction did not reveal any study.
The estimation of oral malodor producing bacteria in tongue and subgingival plaque of chronic periodontitis patients has not been attempted till now. Therefore the aim of this study was to assess the oral malodor using tanita device and organoleptic method and to quantitate odoriferous microorganisms such as P. gingivalis, T. forsythia and F. nucleatum of tongue coating and subgingival plaque using PCR technique in chronic periodontitis patients
Methodology
Following protocol approved by RGUHS, Karnataka, India and the provision of informed consent, 30 chronic periodontitis subjects for this study were recruited from the out patient department of Periodontics, College of Dental Sciences, Davangere, Karnataka, India. Patients of both the sexes were included within the age limit of 30-60 years. Ethical clearance was obtained from the ethical committee of College of Dental Sciences, Davangere, Karnataka, India.
The chronic periodontitis patients with a plaque index of ≤ 2, gingival index of ≤ 2, GBI of 50-80% and periodontal pockets with radiographic presence of bone loss were included in the study. The exclusion criteria were patients suffering from any systemic disease (eg: chronic renal failure, cirrhosis of liver, gastrointestinal disorder, respiratory dysfunction and various carcinoma etc), which were known to cause oral malodor; who had received any antibiotic therapy in the last 3 weeks; who had received any surgical or non-surgical therapy, 6 months prior to the start of the study; who were pregnant or lactating and who were smokers. An observational, cross-sectional, double blind study was performed on a total of thirty patients with chronic periodontitis. Patients were requested to refrain from oral activities, including drinking, eating, chewing gum, and mouthrinsing 2 hours prior to their appointment. A single operator who recorded the oral malodor by both techniques and the microbiologist who performed the PCR analysis were blinded. The clinical parameters recorded were; plaque index [12], gingival index [2], gingival bleeding index [13].
Recording of oral malodor: Organoleptic examination which was considered as a reference standard for oral malodor detection was done. Tanita breath alert, a small hand held breath checking device manufactured by Tanita Corp, Inc, Japan, was used to detect the volatile sulphur compounds (VSCs) and hydrocarbon gases in mouth air. The odor levels were measured by one of the following values on the graphic display of the instrument. 1- No odor; 2- Slight odor; 3- Moderate odor; 4- Strong odor. If no number appeared then it was considered a reading error and the procedure was repeated. After examination of every patient the air opening was cleaned with a dry cloth and the unit was waved gently 4 to 5 times in the air to remove any odors or moisture left in the unit.
Tongue coating assessment: The amount of coating on the tongue’s dorsal surface was estimated by visual examination as follows: 0 – Non visible; 1 – Less than 1/3rd of tongue dorsum surface covered; 2 – Less than 2/3rd covered; 3 – More than 2/3rd covered [14].
Tongue sample and subgingival plaque collection: Tongue Sample was taken from the dorsal surface of the tongue with a wooden spatula [15]. The portion of the swab that contains the coating was dispensed in separate vials containing transport media viz. TE buffer (10ml Tris-HCL, 1ml EDTA pH 8) and Thioglycolate broth. The vial was closed and labelled. The labelled vials were sent to the microbiological laboratory within 24 hours of collection. Pooled subgingival plaque was collected using curette.
Microbial analysis: The pooled subgingival plaque and tongue samples collected in eppendorf vials containing transport media viz. TE buffer (10ml Tris-HCL, 1ml EDTA pH 8) and Thioglycolate broth were sent to the microbiological laboratory within 24 hours of collection, where they were subjected to Multiplex Polymerase Chain Reaction (PCR) analysis. The Pg, Tf and Fn were analysed by PCR analysis. The samples were stored at -2000C upon receipt in the laboratory until DNA extraction and multiplex PCR were performed.
Statistical Analysis
The results obtained from various parameters were subjected to statistical analysis. Results were expressed as mean ± SD and proportions as percentages. Since the measurements are in scores (gradings) and counts, non-parametric tests were used for analysis. Spearman’s correlation coefficient was used to assess the relationship between clinical and microbial parameters.
Results
The study included 30 chronic periodontitis patients of which 15 were males & 15 were females with age range from 30 – 60 years. The results of the study are presented in [Table/Fig-1,2,3&4].
The tongue coating assessment and VSC levels Values are expressed as Mean ± Standard Deviation
| Parameter | Score | No. (%) |
|---|
| Tongue coating assessment | 1 | 1 (3.3) |
| 2 | 11 (36.7) |
| 3 | 18 (60.0) |
| Mean ± SD | 2.5 ± 0.6 |
| Median (Range) | 3.0 |
| Tanita score | I | 5 (16.7) |
| II | 18 (60.0) |
| III | 6 (20.0) |
| IV | 1 (3.3) |
| Mean ± SD | 2.1 ± 0.8 |
| Median (Range) | 2.0 |
| Organoleptic assessment | 3 | 11 (36.7) |
| 4 | 15 (50.0) |
| 5 | 4 (13.3) |
| Mean ± SD | 3.8 ± 0.7 |
| Median (Range) | 4.0 |
Spearman’s correlation of Tongue coating, tanita score, organoleptic score and Clinical parameters
| Measurement | |
|---|
| p-value* | p-value |
|---|
| TC Vs TS | 0.11 | 0.56 |
| TC Vs OS | 0.27 | 0.16 |
| PI Vs TS | 0.14 | 0.48 |
| PI Vs OS | 0.29 | 0.12 |
| GI Vs TS | 0.12 | 0.51 |
| GI Vs OS | 0.26 | 0.17 |
| GBI Vs TS | 0.06 | 0.52 |
| GBI Vs OS | 0.22 | 0.25 |
TC – Tongue Coating; TS – Tanita Score; OS – Organoleptic Score
PI – Plaque Index; GI – Gingival Index; GBI – Gingival Bleeding Index
*Spearman’s Correlation Coefficient p>0.05, Not Significant
Spearman’s correlation of tongue microbial profile, tanita score and organoleptic score
| Measurement | Tongue sample |
|---|
| p-value* | p-value |
| TS Vs Pg | 0.22 | 0.25 |
| TS Vs Fn | 0.13 | 0.50 |
| TS Vs Tf | 0.06 | 0.76 |
| OS Vs Pg | 0.26 | 0.17 |
| OS Vs Fn | 0.22 | 0.24 |
| OS Vs Tf | 0.05 | 0.76 |
TC – Tongue Coating; TS – Tanita Score; OS – Organoleptic Score
PI – Plaque Index; GI – Gingival Index; GBI – Gingival Bleeding Index
Pg- Porphyromonas gingivalis, Fn- Fusobacterium nucleatum, Tf- Tannerella forsythia *Spearman’s Correlation Coefficient p>0.05, Not Significant
Subgingival and Tongue Microbial Profile in Chronic Periodontitis Patients
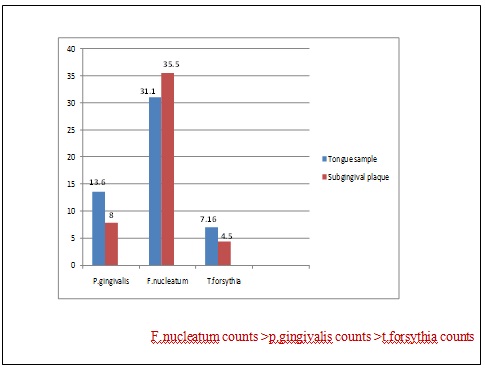
The mean plaque index of 1.91 ± 0.26, gingival index of 1.72 ± 0.25 and GBI of 69.72 ± 23.54 were demonstrated in the selected patients. The tongue coating score of 3 was found in 60% of selected subjects. The VSC levels representing halitosis, measured using tanita breath alert demonstrated score II in 60% of selected subjects while organoleptic score of 4 was found in 50% of subjects [Table/Fig-1].
In tongue samples, highest presence of Fn (31.1±36.5) was followed by Pg (13.6±13.3) and Tf (7.16±8.68) similar to subgingival plaque sample [Table/Fig-4]. Considering the spearman’s correlation, a weak positive correlation was found between VSC levels (tanita & organoleptic score) to clinical parameters [Table/Fig-2]. The spearman’s correlation coefficient between tanita score vs Fn, Pg, Tf: organoleptic score vs Fn, Pg, Tf were not significant [Table/Fig-3].
Discussion
Humans emit a variety of volatile and nonvolatile molecules that are influenced by genetics, diet, stress and disease. Halitosis, from the Latin word for breath ‘halitus’ is a complaint analogous to body odor [16], is used to describe any disagreeable odor in the breath. Halitosis that frequently causes embarrassment, may also affect the interpersonal social communication [17]. It has, further, led to the establishment of the pharmacological and cosmetic industries (with millions of pounds spent annually on medications and over the counter products). The true prevalence of halitosis is unknown and some reports are difficult to be evaluated unless they specify the classification, terminology and methodology used [18].
However, the available evidence suggests that halitosis is common and can affect people of all ages. The prevalence of persistent oral malodor as revealed by a recent Brazilian study, was reported to be 15%. It was nearly three times higher in men than in women (regardless of age) and the risk was slightly more than three times higher in people over 20 years of age than those below 20 years of age, controlling for gender [19]. Majority of studies report that about 30% of people have positive halitosis [20–22]. Only a few studies state that more than 50% of the population has positive halitosis [23]. Persistent halitosis occurring as a result of intraoral causes usually originates from the posterior dorsum of the tongue and/or oral/dental diseases, including periodontal disease, and can be severe enough to be considered socially unacceptable (also known as pathologic halitosis) [18]. The sufficient information provided by the studies during 1970’s, enabled us to determine that the major cause of bad breath is oral microflora which produces volatile odoriferous molecules, including sulphur compounds and organic acids [24,25].
Several methods have been developed to identify these microorganisms, many of which are polymerase chain reaction (PCR) based on bacterial detection systems. Most of the reported PCR based diagnostic systems are qualitative analysis methods and are therefore, unsuitable for the accurate evaluation of bacteria causing oral malodor. Quantitative analysis is essential for monitoring the cell number and the ratio of bacteria in oral specimens from the saliva, tongue coat, and subgingival plaque [23]. So a quantitative hot-start, multiplex PCR was utilised in this study.
Systemic pathological states, such as diabetes mellitus, uremia and hepatic diseases, induce metabolic products that are detectable as oral smells. It is always easy to recognise halitosis, but identifying the exact cause is more complex. The clinical labelling and interpretation of different oral malodors contribute to the diagnosis and treatment of underlying disease wherein treatment is directed at the underlying cause.
The current study results are discussed below: The selected chronic periodontitis patients had plaque index of ≤ 2, gingival index of ≤ 2 and gingival bleeding index of 50-80%.
In tongue coating assessment, 60% patients showed more than 2/3 of the tongue dorsum to be covered (score of 3). In tanita score, 60% patients presented with slight odor (score II). In organoleptic assessment, 50% patients presented with strong offensive odor (score 4). The tongue microbial profile of the tongue dorsum showed maximum counts of F.nucleatum (31.1) followed by P.gingivalis (13.6) and T.forsythia (7.16). The pooled subgingival plaque sample also demonstrated a similar pattern with the highest value of Fn (35.5) followed by Pg (8) and Tf (4.5) [26].
Both the tanita & organoleptic scores & tongue microbial profile showed a weak positive correlation with tongue coating. The Fn count in tongue coat was found be lower than in subgingival plaque [27]. In contrast, Pg was identified in one sample [28]. and found to be less in scrapings from the tongue surface [27].
The results of the study are compared with the available literature. In this study, a weak positive correlation was demonstrated between clinical parameters (TI, PI, GI, GBI) and tanita & organoleptic scores using Spearman’s correlation. A positive correlation between volatile sulphur compounds (VSC) and Tongue coating has been demonstrated [28–33].
In the present study the comparison of microbial profile with VSC levels showed a weak positive correlation of Pg, Fn and Tf count with organoleptic and tanita score. A quantitative analysis of P. gingivalis, F. nucleatum, T. forsythia and T. denticola in the saliva, on the tongue coating, and in the subgingival plaque of patients with oral malodor using real time PCR showed correlation between increased VSC levels and increased levels of Pg and Tf in subgingival sample and Fn in tongue coating. In a study, the association of oral malodor and tongue periodontal pathogens (Pg, Tf, Pi, Pn, Td) with real time PCR has been reported [34]. Among the periodontopathogens, Tf displayed higher proportions in malodor subjects than healthy controls. In contrast, it was concluded that there was no obvious association between halitosis & any specific bacterial genus in dorsum of tongue [35]. The increased species diversity found in halitosis samples suggests that halitosis may be the result of complex interactions between several bacterial species. Recent studies have indicated that the dorsal surface of the tongue may be the primary source of microbial putrefaction in mouth [3,28]. Tongue coating is an important factor in the formation of oral malodor in both periodontally diseased & healthy people. Indeed, studies suggest that the flora on the tongue is similar to odor producing periodontal bacteria [28]. The dorsal surface of the tongue is an important factor in the development of halitosis regardless of the periodontal status. Only few data exist on the types of bacteria present on the tongue surfaces of people with subjective complaints of oral malodor [28]. The role of specific bacteria on the tongue surface in malodor production has not been fully understood in vivo. Several studies report significant association of BANA positive organisms i.e; P.gingivalis and B. forsythus with the oral malodor [15,36–38,3,27,34,24].
Previous studies have reported the association between VSC levels in mouth air and periodontal pathogenic bacteria detected by the benzoyl –DL- arginine-naphthylamide (BANA) test at various oral sites. However the specific role of the bacterial species Pg, Td or Bf in the production of oral malodor could not be detected by BANA [36].
It has also been suggested that the presence of B. forsythus, P. gingivalis and P. intermedia influenced the production of VSC. In a yet another study, it was concluded that gram positive bacteria contributed little to oral malodor production whereas gram negative bacteria produce large amounts of VSC; among the gram negative bacteria, P. gingivalis, P. intermedia, F. nucleatum and T. denticola, the periodontopathogens are the major contributors [24].
There was a large variability in the counts of individual species in subgingival and tongue samples. This finding is consistent with previous studies of the tongue flora, which showed similar fluctuations in total count and in the prevalence and proportions of recovered individual bacterial species [28]. The variability may be explained in part by the difficulty in obtaining a standardized sample of the tongue flora. The tongue is a soft, highly mobile and flexible structure, which hampers standardization of sampling techniques, such as pressure used during collection and surface area being scraped [28]. Fluctuations in the tongue flora over time within an individual as observed by a few authors may provide an additional explanation for the variability in bacterial counts [39]. Since the early history of medicine, breath analysis has been used as a diagnostic tool, as evidenced by comments on breath odors that were characteristic of particular diseases. Some breath odors are part of the everyday medical vocabulary, such as fetor hepaticus, uremic breath, and diabetic breath. The present available information supports the use of breath analysis for the diagnosis of exposure to volatile organic solvents and anesthetics as a preferred method over blood and urine analysis. Generally, analysis of blood and urine samples for these compounds are more cumbersome and tedious.
The clinical implications of the present study are, the dentist/periodontist should emphasise on tongue cleaning measures that would reduce the odoriferous microbial load. The tanita breath alert which is a simple instrument could be a part of home care measures to assess the malodor levels by patient himself.
Further studies are being conducted as recently the importance of oral malodor has been recognised as it carries considerable social stigma in our modern society. Quantification of VSC-producing bacteria is important for diagnosis and therapeutic assessment of oral malodor. Conventional tests, gas chromatography, organoleptic tests and portable sulfide monitors are essential tools for evaluating oral malodor. However, these tests cannot measure any direct correlation between the disease and pathogenic bacteria. In addition to these conventional tools, PCR will support diagnosis of oral malodor pathogens and contribute to control of VSC production [11]. The limitation of the present study was that the Gas chromatography was not utilized for quantitative estimation of individual odoriferous compounds because of non availability and cost factor.
The dental research community has ignored for a long period the subject of oral malodor. Recently, along with the growing public and media interest in oral malodor, dental professionals are becoming more aware of their patient’s concern/needs. It is our hope that future studies will overcome the difficulty of diagnosing this long standing problem and provide effective treatments to relieve individuals who suffer from oral malodor.
Conclusion
In Tongue coating assessment 60% patients showed more than 2/3 of the tongue dorsum to be covered (score of 3). In Tanita score 60% patients presented with slight odor (score 2). In Organoleptic assessment 50% patients presented with strong offensive odor (score 4). The microbial profile of the tongue dorsum showed maximum counts of F.nucleatum followed by P.gingivalis and T.forsythia. A weak positive correlation exists between VSC values (tanita and organoleptic) and tongue coating and VSC values and microbial profile in tongue samples.
TC – Tongue Coating; TS – Tanita Score; OS – Organoleptic Score
PI – Plaque Index; GI – Gingival Index; GBI – Gingival Bleeding Index
*Spearman’s Correlation Coefficient p>0.05, Not Significant
TC – Tongue Coating; TS – Tanita Score; OS – Organoleptic Score
PI – Plaque Index; GI – Gingival Index; GBI – Gingival Bleeding Index
Pg- Porphyromonas gingivalis, Fn- Fusobacterium nucleatum, Tf- Tannerella forsythia *Spearman’s Correlation Coefficient p>0.05, Not Significant