Effectiveness of Cryogen Tetrfluoroethane on Elimination of Gingival Epithelium and its Clinical Application in Gingival Depigmentation–Histological Findings and Case Series
Santhosh Kumar1, G. Subraya Bhat2, K. Mahalinga Bhat3
1 Assistant Professor, Department of Periodontics, Manipal College of Dental Sciences, Manipal, Udupi, Karnataka, India.
2 Professor and Head, Department of Periodontics, Manipal College of Dental Sciences, Manipal, Udupi, Karnataka, India.
3 Professor, Department of Periodontics, Manipal College of Dental Sciences, Manipal, Udupi, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Santhosh Kumar, KMC Qts No – 120, Madhava Nagar, Manipal – 576104, Udupi District, Karnataka, India.
Phone: 9741968550,
E-mail: drsanthoshkumar@gmail.com
Objective: To histologically assess and clinically co-relate the effectiveness of cryogen Tetrafluoroethane (TFE) for gingival depigmentation procedure.
Material and Methods: Twelve patients having unaesthetic gingival melanin pigmentation were included in the study. Gingival tissues of eight patients having gingival melanin pigmentation undergoing gingivoplasty or gingivectomy for crownlengthening were exposed to the cryogen and this was used for the histological examination. Gingivectomies were done after 8, 24, 96 hours and after a week of application of tetrafluoroethane. Four fair skinned patients complaining of unaesthetic gingival hyperpigmentation underwent gingival depigmentation using Tetrafluoroethane cryogen.
Results: Histologically after 96 hours of application of cryogen there was complete loss of retepegs and epithelial detachment from the corium was evident. Complete re – epithelialisation was noted after a week and was clinically correlated.
Conclusion: We therefore, concluded that histologically tetrafluoroethane can effectively destroy gingival epithelium without causing damage to the connective tissue and clinically the color of the gingiva had more pleasing appearance 6 months postoperatively. Hence the cryogen can be used safely for depigmentation procedure.
Cryosurgery, Depigmentation, Epithelial detachment, Liquid nitrogen, Gingiva, Tetrafluoroethane
Introduction
Low temperature was commonly used to achieve specific effect on tissues and its popularity has increased from the past 3–4 decades [1]. Cryosurgery is an effective method of tissue destruction by freezing and has become firmly established surgical technique for treatment of diverse benign and malignant cutaneous lesions [2] and it is also used in dental practice [3].
In 1969, Heitmann, Dorman and Collins [4] reported that the gingiva freezes at a temperature ranging between 14.20C to a high of 31.8oC may be conducted without adversely affecting the vitality of adjacent teeth. With a melting point of-101°C and a boiling point of -26°C, Tetrafluoroethane is commercially available in a pressurized spray can and immediately evaporates without residue following spraying. This gaseous fluorocarbon is used in dentistry in the field of endodontics for cold-pulp testing for full crowned or natural teeth and in orthodontic treatment to facilitate the seating of nickel-titanium expansion loops [5].
Tetrafluoroethane is safe, exposure via short term inhalation and skin contact show no effect on inhibition pulse, blood Pressure, electrocardiogram or lung function in healthy volunteer [6]. Tetrafluoroethane exerts no adverse effect on development, maturation and reproduction and does not have genotoxic and oncogenic capacity on animals [7–9].
The cryogen used in this study was obtained in a pressurised can and dispensed via outlet nozzle from the can. The present study attempted to histologically assess and clinically co-relate the effectiveness of ultralow temperature using Tetrafluoroethane in elimination of gingival epithelium in turn resulting in depigmentation.
Material and Methods
Twelve patients were used as experimental subjects for the study.
Clinically all were free of any form of gingivitis.
A detailed medical history which included pregnancy, breast feeding,systemic diseases which were associated or not associated with the gingival melanin pigmentation, medications and adverse drug reactions were taken into consideration. The relative contraindications included cold intolerance and cold utricaria.
Marginal gingiva including the interdental papillae of the maxillary cuspid and bicuspids of patients undergoing gingivoplasty or gingivectomy for crown lengthening and had melanin pigmentation were frozen.
A total of eight gingivae obtained from gingivectomy for crown lengthening were frozen.
Freezing procedure: a. The tissue to be frozen was isolated, dried; topical anaesthetic (10% Xylocaine, Andheri east, Mumbai, India) was sprayed.
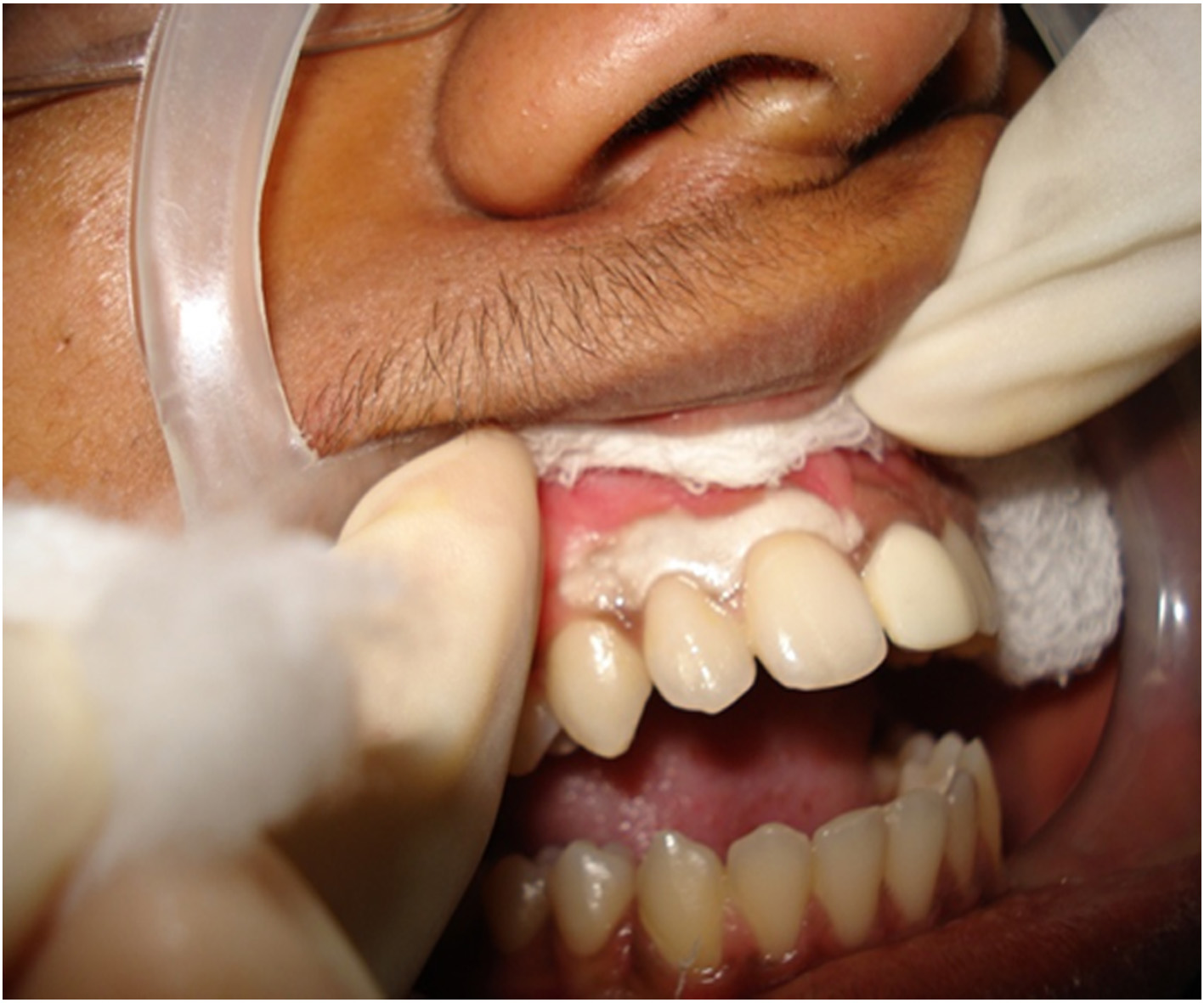
b. The cryogen was dispensed in a kidney tray and was transferred to the site using a cotton pledget carried using a tweezer. It was applied for 2-3 minutes without applying too much of pressure [Table/Fig-1A].
c. Thawing occurred within 10-12 seconds after removal of the cotton pledget from the site.
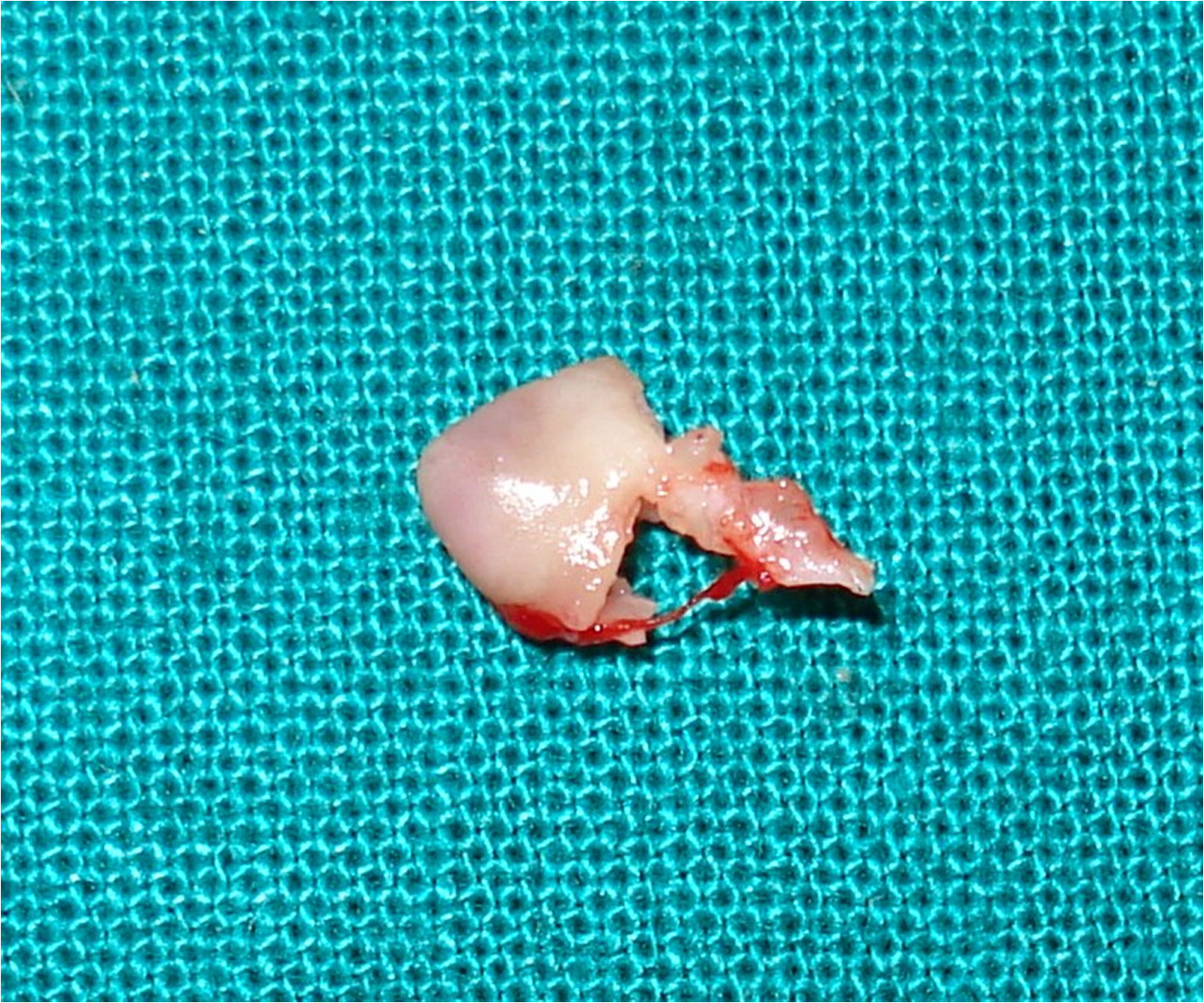
Biopsies were taken in the following order: 8, 24, 96 hours and one week after freezing [Table/Fig-1B].
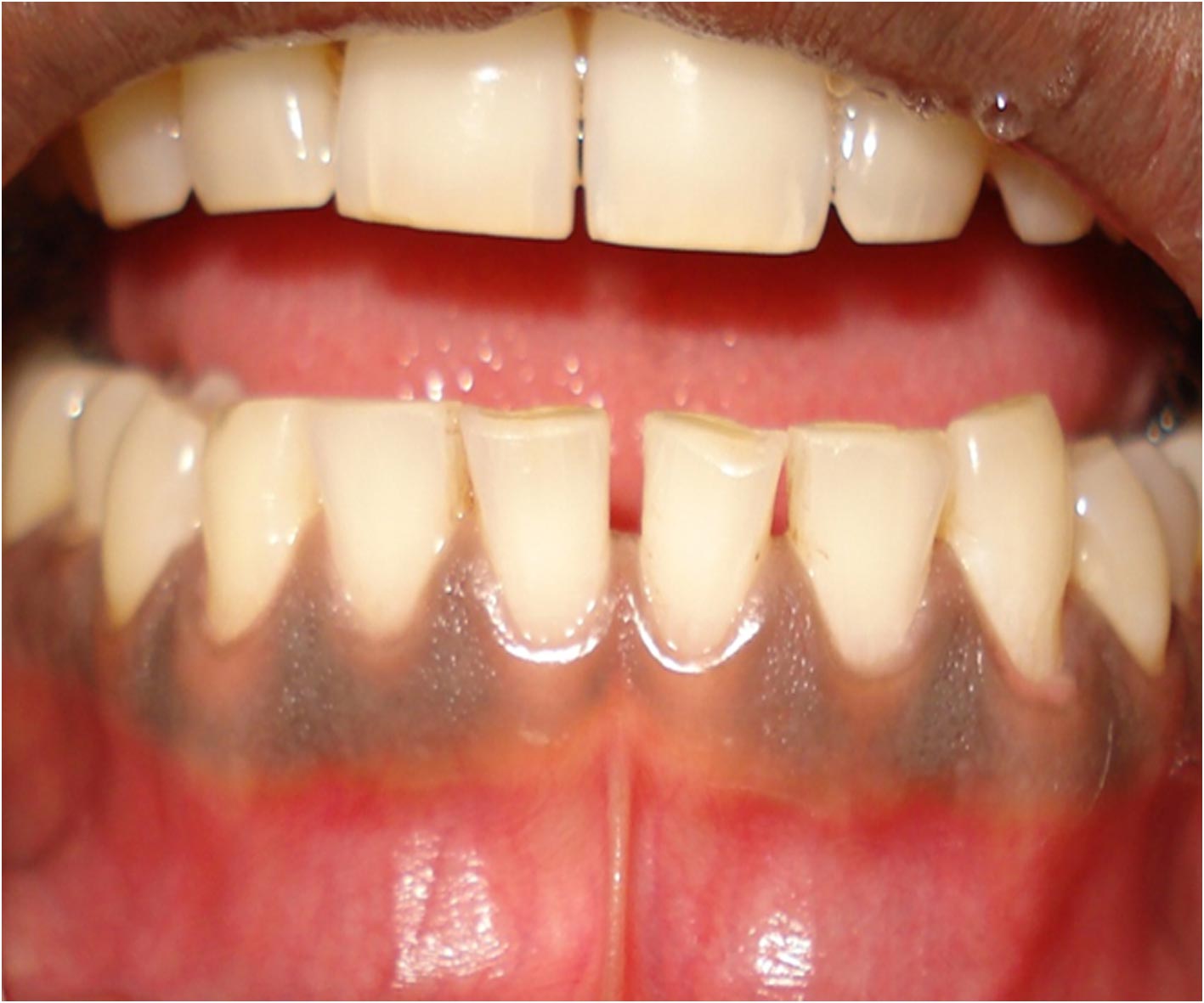
The patients undergoing cryosurgical depigmentation had generalized diffused pigmentation score of 3 classified according to the gingival pigmentation index (GPI) [10] as shown below. The patients were reviewed after 6 months of depigmentation.
Application of the cryogen
Excised tissue sent for histological examination
Score Criteria
0 Absence of pigmentation, pink color of the gingiva
1 Spots of brown to black
2 Brown to black pigmentation, more than spots but not diffuse (patches of pigmentation)
3 Diffuse brown to black pigmentation involving papillary, marginal and attached gingiva
Results
Clinical signs
Immediately after the application of the cryogen a solidly frozen area resembled frozen tissue. Thawing was evident and recognized as the size of the frozen area decreased and completely disappeared in 15-20 seconds. The appearance of site after thawing was indiscernible from adjacent site with slight redness.
After 8 hours the site showed shallow elevated surface and in 96 hours the overlying epithelium easily peeled off exposing the underlying connective tissue. After a week of application the overlying epithelium was completely replaced by a depigmented epithelium.
Histological findings
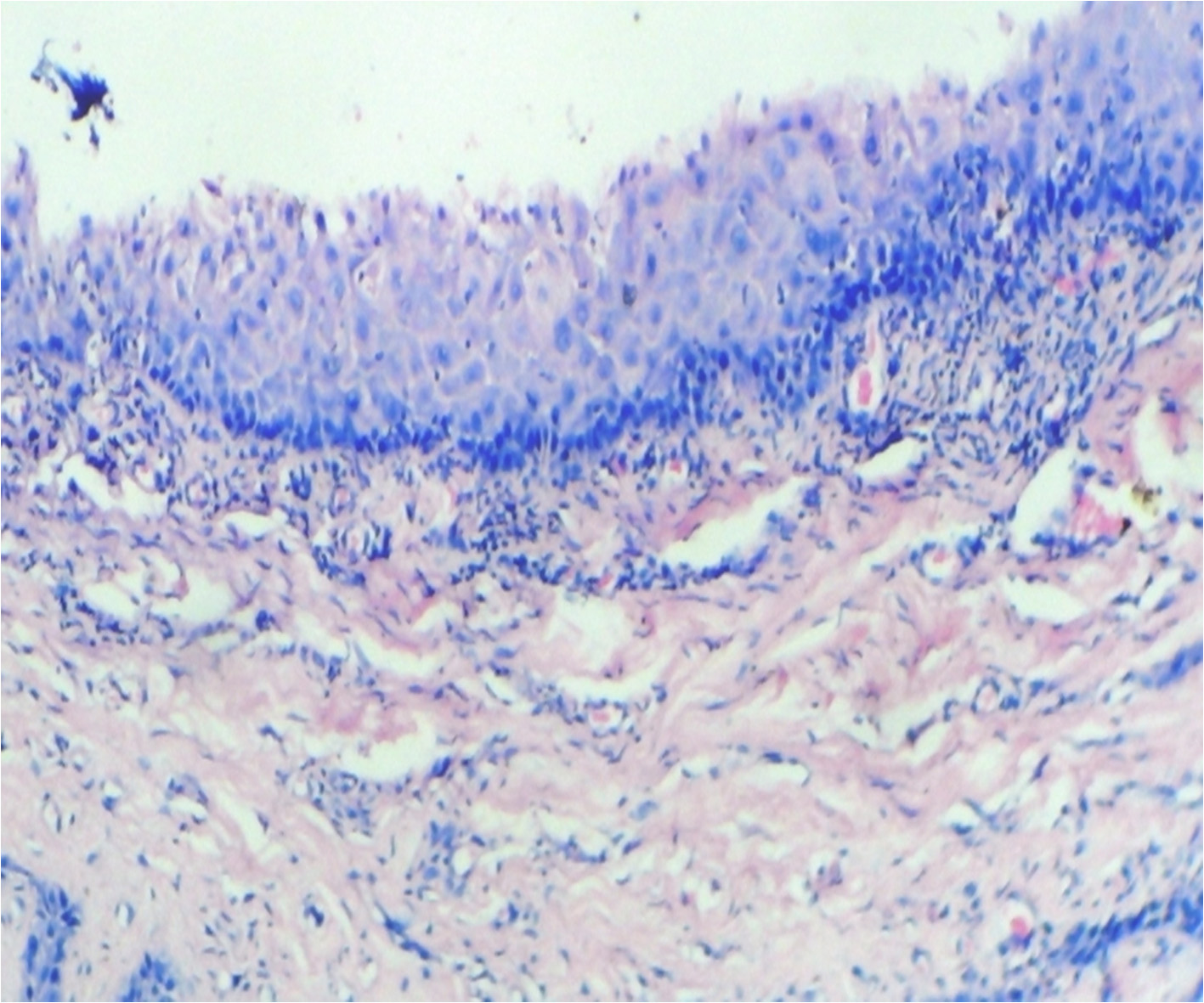
After 8 hours [Table/Fig-2A] on examination the para keratinized stratified squamous epithelium showed loss of retepegs. There were some degenerative changes in the form of necrotic material. Basement membrane was inconspicuous. Underlying connective tissue stroma showed severe chronic inflammatory response mainly in the form of lymphocytes and plasma cells. After 24 hours histological examination of the specimen showed parakeratinised stratified squamous epithelium with acanthosis at places. The epithelial cells show desquamative changes in the form of hydrophic degeneration [Table/Fig-2B]. Underlying connective tissue stroma is loose and oedematous with few chronic inflammatory cells in the form of lymphocytes and plasma cells.
Eight hours following freezing showing epithelial degeneration
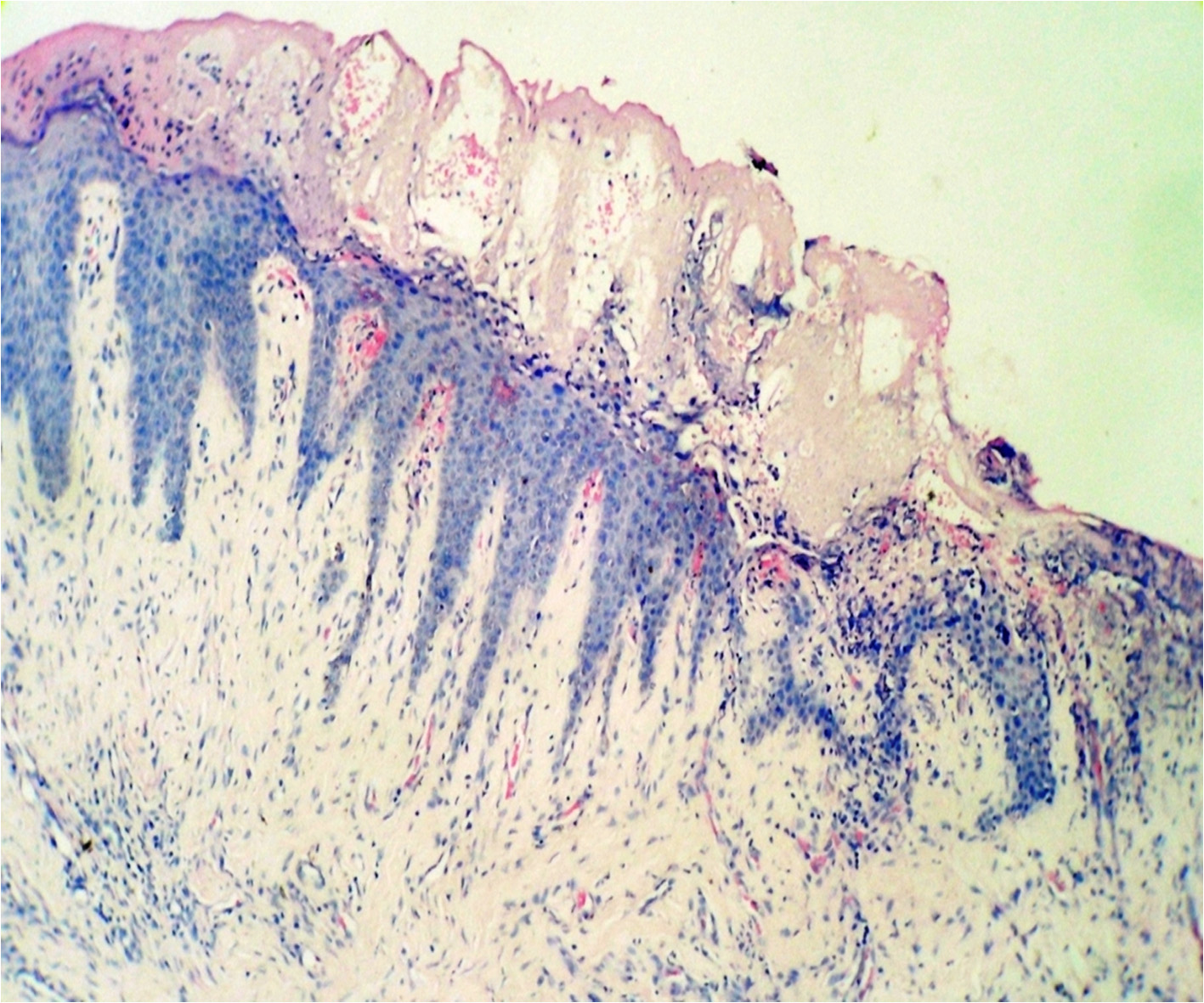
Specimen after 24 hours showing loss of retepegs
After 96 hours the soft tissue showed parakeratinised squamous epithelium exhibiting degeneration. Underlying connective tissue is delicate to dense with focal aggregate of chronic inflammatory cells [Table/Fig-3A].
Four day specimen showing complete loss of retepegs and subepithelial cleft
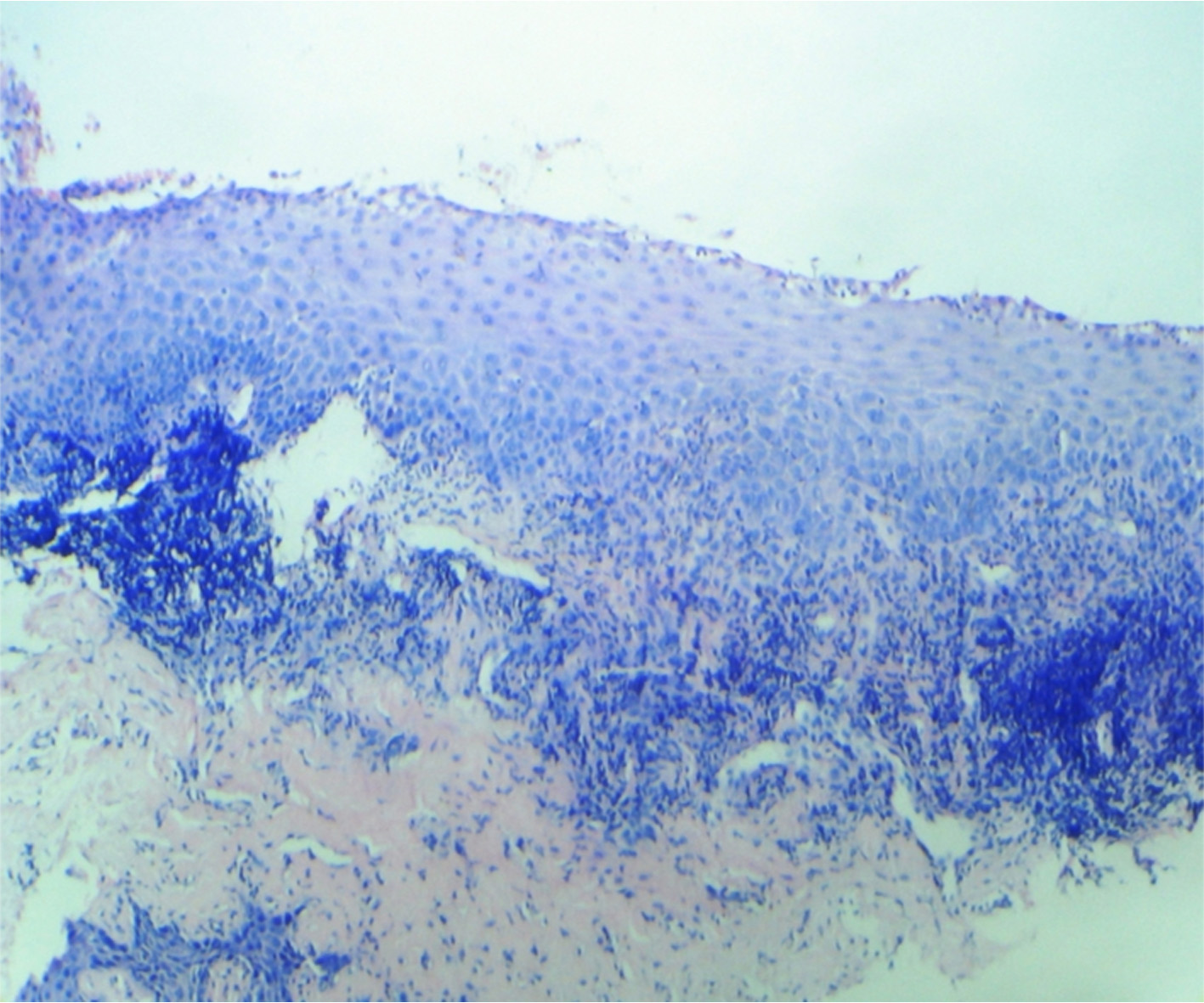
Clinically the gingival tissue surface showed desquamation and redness suggesting healing and replacement with a lighter colored gingiva [Table/Fig-3B] the pigmentation of the gingiva changed to a score of 0 in three out of four cases and score of 1 in one case.
Clinical resemblance after four days of application
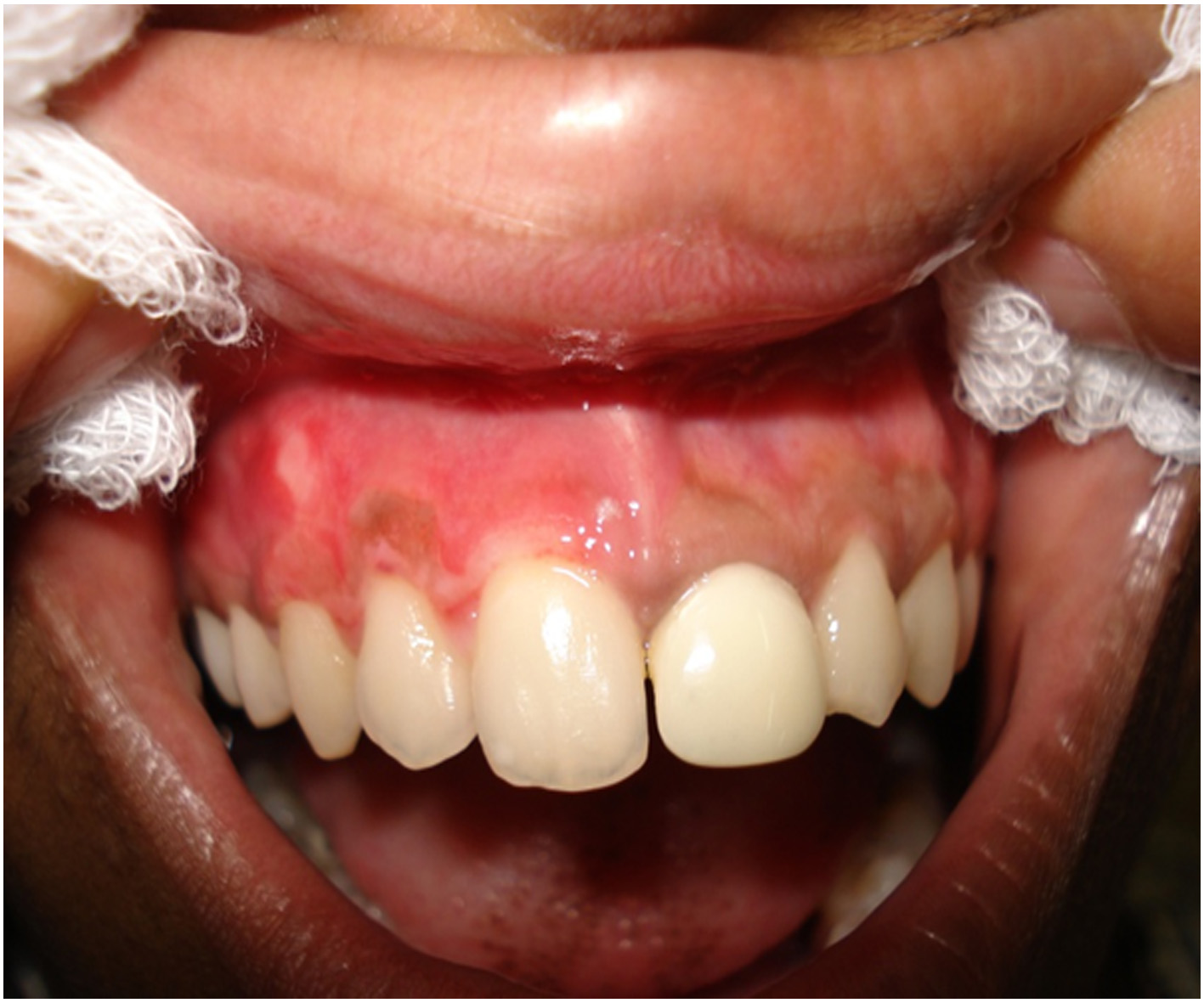
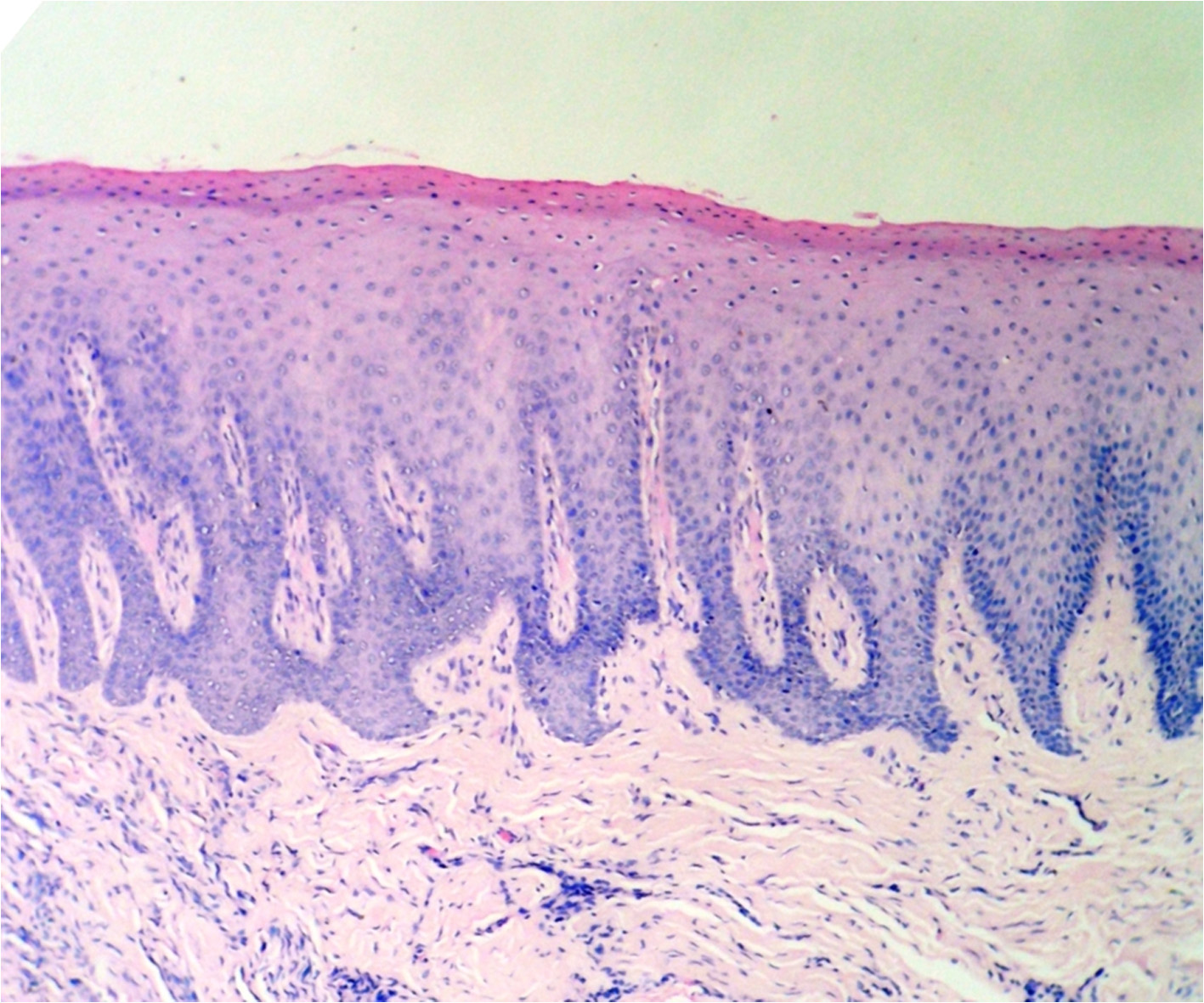
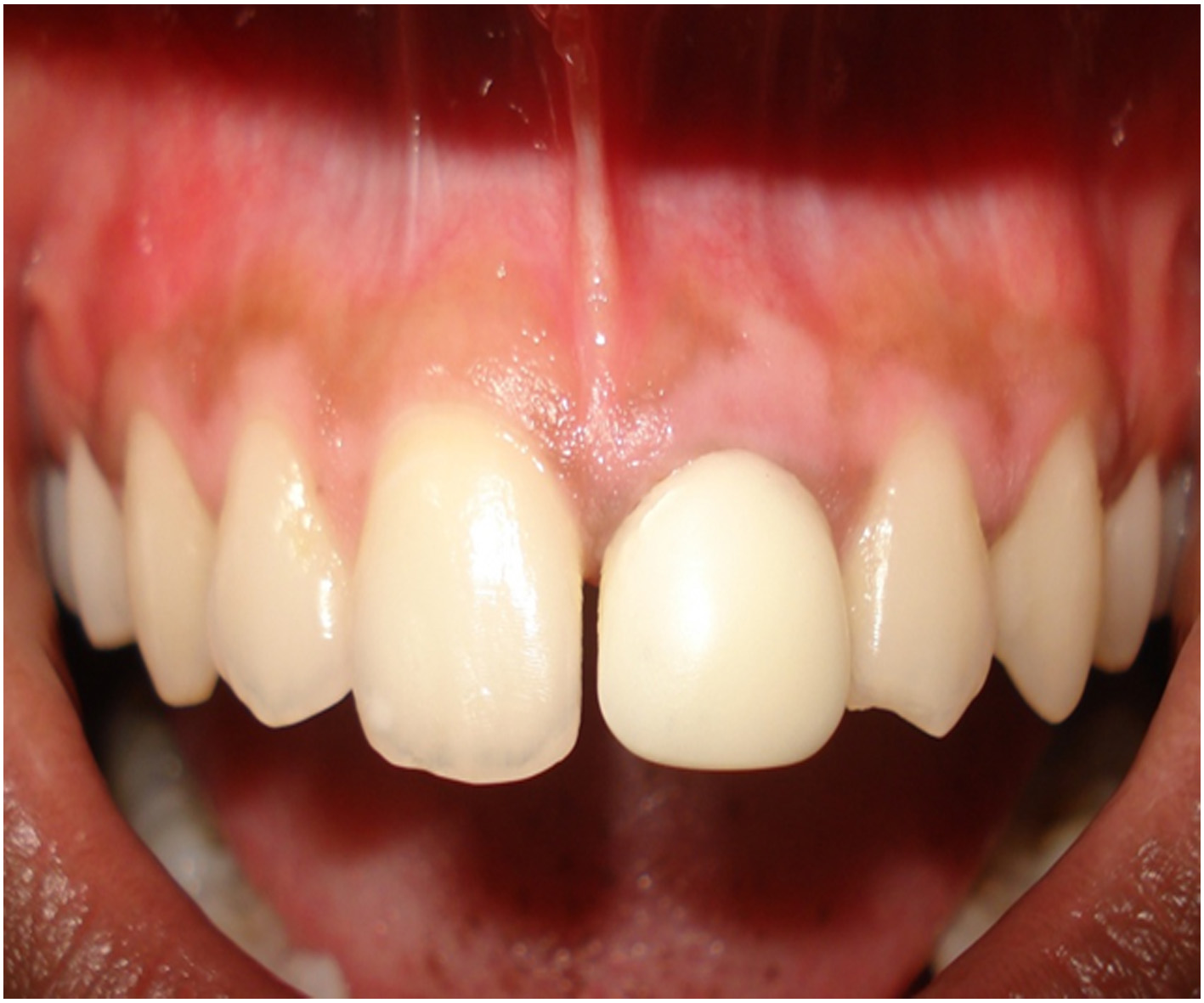
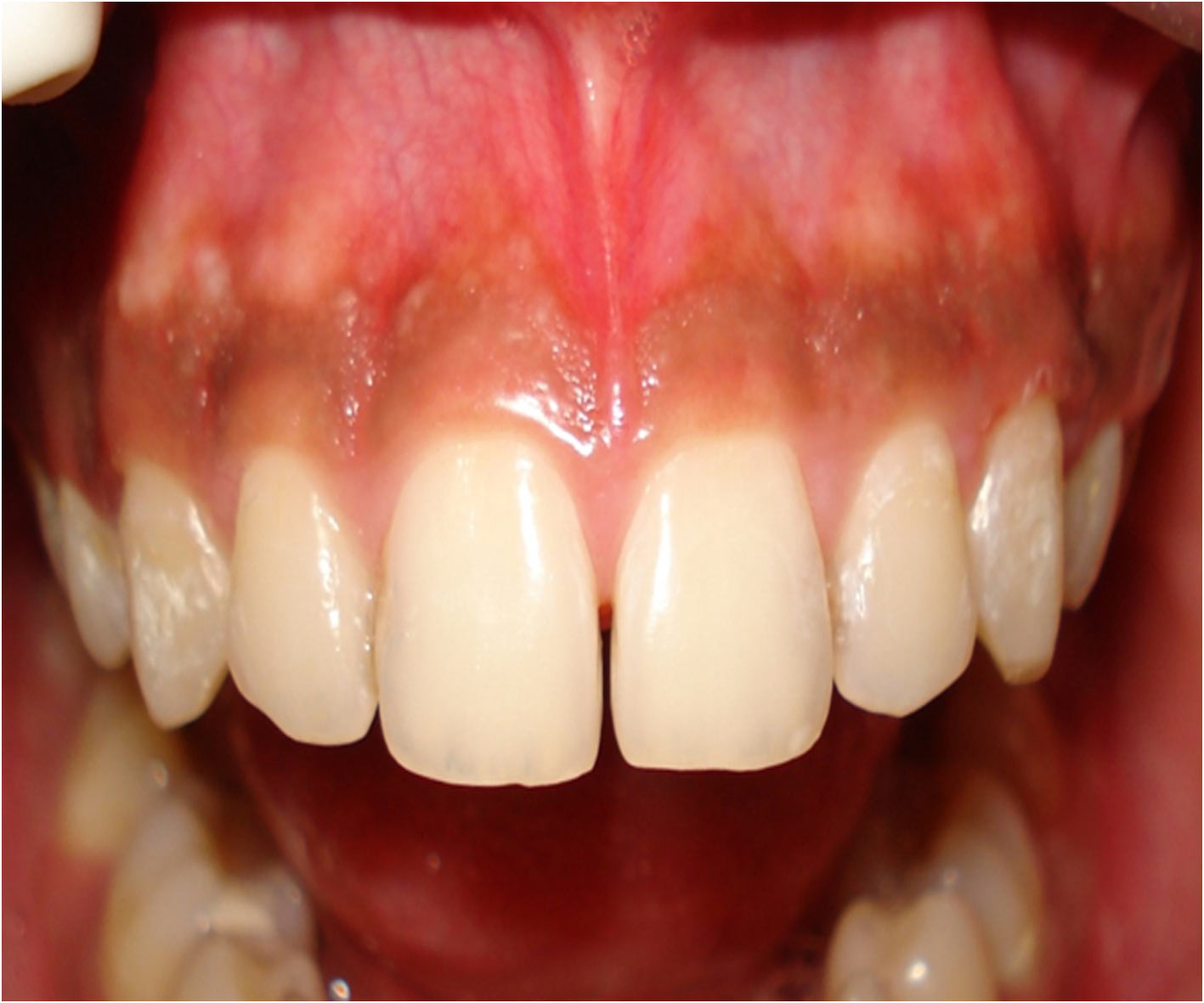
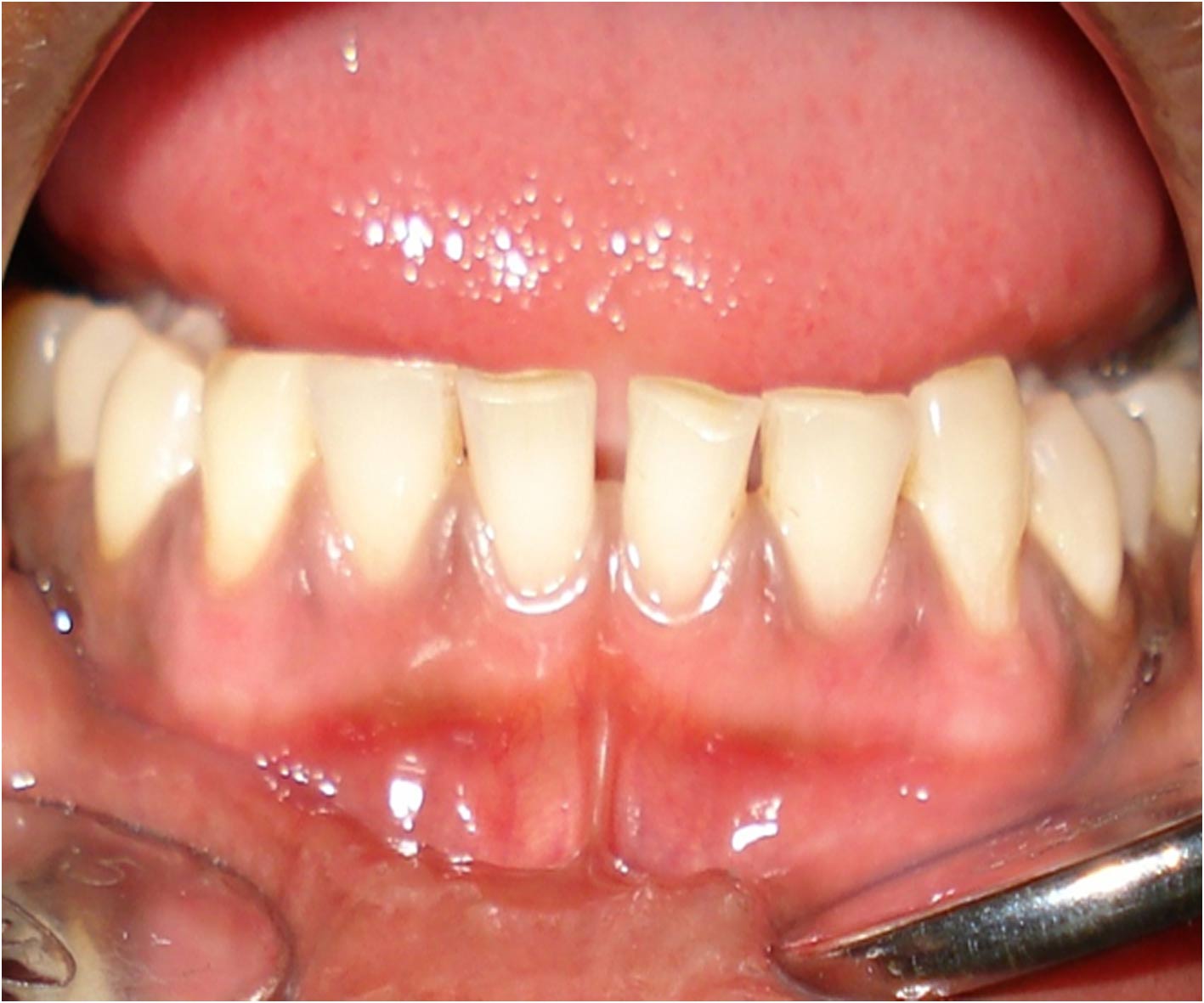
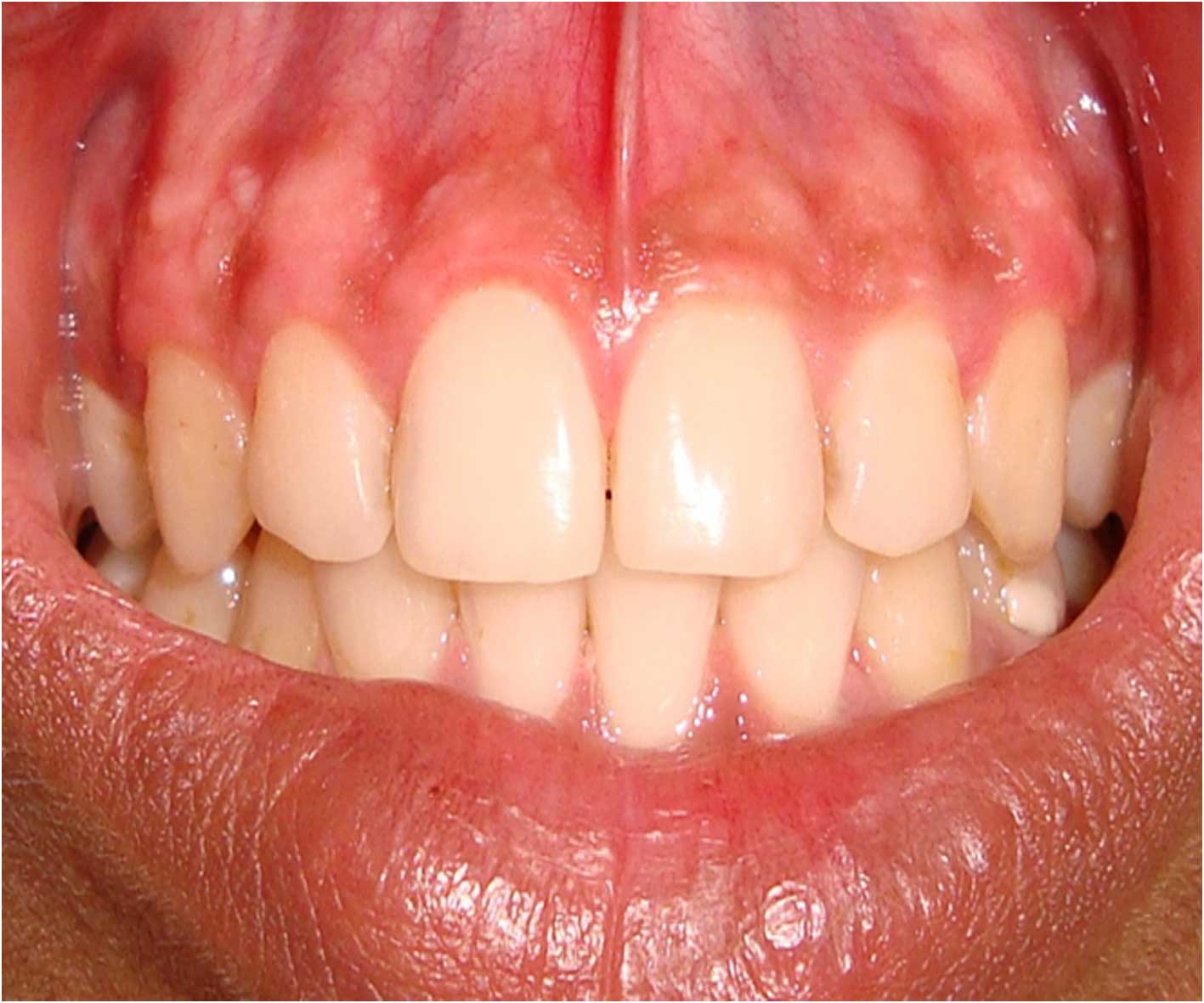
After a week of application the cryogen there was complete reepithelialisation [Table/Fig-4A] and formation of retepegs with absence of chronic inflammatory infiltrate in the connective tissue. Patients with pigmentation were treated using this technique and the results showed clinical applicability of this material [Table/Fig-4B]. Following 6 months of treatment there was clinically visible changes as compared to the results after depigmentation [Table/Fig-5A, 5B] and [Table/Fig-6A, 6B].
Specimen after a week. Showing complete reepithelialisation
Clinical resemblance after a week of application of cryogen
Patient with pigmentation in the mandibular anterior region
Patient with pigmentation in the maxillary anterior region
Postoperative photograph after 6 months following gingival depigmentation
Postoperative photograph after 6 months following gingival depigmentation
Discussion
In the present study using ultralow temperature cryogen tetrafluoroethane on gingival biopsy showed that after 8 hours of application the epithelium showed degenerated retepegs, and was elevated from the underlying connective tissue; this finding is similar to the finding in the study done by Mayer P D, Gerald Tussing, Frank M Wentz [11].
Studies have shown that superficial tissue necrosis occurs first after the cryosurgery then rapid epithelial migration covered the area [3]. Epithelial migration covers the denuded connective tissue and regenerate rapidly after a week of application. The stability of the depigmented area after six months of application of the cryogen is similar to the results seen in other studies [10, 12].
Gas expansion system used in cryosurgery is expensive and it is not widely used [10]. Cryosurgical treatment of oral lesions by liquid nitrogen [13,14] has been reported but both the gas expansion cryosurgical system and liquid nitrogen applied with cotton swabs are not easily obtainable in most clinics. Cross infection is also a disadvantage of using dipstick method [15].
In contrast to laser surgery and the conventional cryosurgery methods, the Tetrafluoroethane cryosurgery serves as an inexpensive, easy-to-use, store and transport [16]. In addition, the lack of bleeding and scar formation, its application without a regional anaesthesia, sutures or dressing, the ease of application of the cryogen at the papillary area by using a small cotton pledget, the added advantage of the depth control by using the time factor [11] makes the Tetrafluoroethane cryosurgery superior to scalpel, Laser and the conventional cryotherapy procedures in many aspects.
Conclusion
The effect of ultralow temperature on the gingival tissue causes the epithelium to undergo cryonecrosis, helps to eliminate gingival melanin pigmentation by controlled epithelial destruction and clinically the gingiva heals without untoward side-effect and would be esthetically pleasing.
[1]. Torre D, Cutaneous cryosurgery: current state of the artJ Dermatol Surg. Oncol 1985 11:292-93. [Google Scholar]
[2]. Kuflik EG, Cryosurgery updatedJ Am Acad Dermatol 1994 31(6):925 [Google Scholar]
[3]. Tal H, Stahl S, Elimination of epithelium from healing postsurgical periodontal wounds by ultralow temperature: initial observationsJ Periodontol 1985 56(8):488-91. [Google Scholar]
[4]. Heitmann Kenneth Dorman Homer L, Collins C K, Temperature recordings in the Dental pulp During CryosurgeryJ. Periodontol 1969 40:655 [Google Scholar]
[5]. Miller SO, Johnson JD, Allemang JD, Strother JM, Cold testing through full-coverage restorationsJ Endod 2004 30:695-700. [Google Scholar]
[6]. Emmen HH, Hoogendijik EMG, Klöpping WAA, Muijser H, Duistermaat E, Ravensberg JC, Human safety and pharmacokinetics of CFC alternative propellants HCF 134a (1,1,1,2- Tetraflouroethane) and HCF 227(1,1,1,2,3,3,3-Hepatoflouropropane) following whole-body exposureRegul Toxicol Pharmacol 2000 32:22-35. [Google Scholar]
[7]. Alexander DJ, Libretto SE, Chevalier HJ, Imamura T, Pappritz G, Wilson J, HFA-134a (1,1,1,2-tetrafluoroethane); lack of oncogenicity in rodents after inhalationHum Exp Toxicol 1995 14:706-14. [Google Scholar]
[8]. Alexander DJ, Libretto SE, Adams MJ, Hughes EW, Bannerman M, HFA-134a (1, 1, 1, 2-tetrafluoroethane): effects of inhalation exposure upon reproductive performance, development and maturation of ratsHum Exp Toxicol 1996 15:508-17. [Google Scholar]
[9]. Collins MA, Rusch GM, Sato F, Hext PM, Millischer RJ, 1,1,1,2-Tetrafluoroethane: repeat exposure inhalation toxicity in the rat, developmental toxicity in the rabbit, and genotoxicity in vitro and in vivoFundam Appl Toxicol 1995 25:271-80. [Google Scholar]
[10]. Singh Vishal, Bhat Subraya, Kumar Santhosh, Bhat K Mahalinga, Comparative evaluation of gingival depigmentation using tetrafluoroethane cryosurgery and lasersClin Adv Periodontics 2012 2(3):129-34. [Google Scholar]
[11]. Mayers Phil D, Tussing Gerald, Wentz Frank M, The histological reaction of clinically normal gingiva to freezingJ. Periodontol 1971 42(6):346-52. [Google Scholar]
[12]. Kumar Santhosh, Bhat G Subraya, Bhat K Mahalinga, Developments in techniques for gingival depigmentation. An updateIndian journal of dentistry 2012 3(4):213-21. [Google Scholar]
[13]. Kumar Santhosh, Bhat G Subraya, Bhat K Mahalinga, Comparative evaluation of gingival depigmentation using tetrafluoroethane and gingival abrasion technique: two year follow-upJ Clin Diagn Res 2013 7(2):389-94. [Google Scholar]
[14]. Toida M, Ishimaru JI, Hobo N, A simple cryosurgical method for treatment of oral mucosal cystsJ Oral Maxillofac Surg 1993 22:353-55. [Google Scholar]
[15]. Yeh CJ, Cryosurgical treatment of melanin-pigmented gingivaOral Surg Oral Med Oral Path Oral Radiol Endod 1998 86:660-63. [Google Scholar]
[16]. Arikan F, Gürkan A, Cryosurgical treatment of gingival melanin pigmentation with tetrafluoroethaneOral Surg Oral Med Oral Pathol Oral Radiol Endod 2007 103:452-57. [Google Scholar]