The protein, p16, is a negative regulator of the cell cycle and it is the product of the cyclin dependent kinase 2 (CDKN2) gene. Studies done on the molecular genetics of oral cancer have shown that the CDKN2 gene was frequently inactivated by methylation or homozygous deletions [1]. Inactivation of p16(INK4a), which was encoded by the CDKN2 gene has been widely associated with oral squamous cell carcinomas [2]. P16 is a cyclin dependent kinase inhibitor that binds to CDK4 and forms a p16-CDK4 complex, which prevents phosphorylation of the product of the retinoblastoma susceptibility gene pRb, and pRb remains in an hypophosphorylated, growth suppressive state. In the case of dysfunction of p16, CDK4 can bind to cyclin D and form a CDK4-cyclin D complex. This complex promotes the phosphorylation of pRb and the release of a transcriptional factor (TF), which accelerates the cell cycle. The inactivation of p16, therefore, leads to deregulation of the cell cycle control and to cell proliferation [3–4]. Perturbation of this cell cycle regulatory pathway by a tumour specific genetic alteration or by inactivation of p16 or pRb or overexpression of CDK4 or cyclin D1, has been seen in many human cancers [5]. The loss of p16 function by gene deletion, methylation and mutation within the reading frame, have been found in various cancers [6,7].
Carcinoma ex-pleomorphic adenoma is a rare histologic subtype of salivary gland cancer, with an overall poor prognosis. Carcinoma ex-pleomorphic adenoma (CXPA) is considered to be a malignant transformation of a pre-existing pleomorphic adenoma [8]. Carcinoma ex-pleomorphic adenomas have been estimated to account for 10% of all salivary gland malignancies [9]. Despite the recognized clinical importance of CXPA, only little is known about its biology and therefore, the diagnosis of CXPA is a challenge for pathologists. The purpose of this study was to determine the alterations in the immunohistochemical expression of p16 in normal tissue of the salivary gland, surrounding carcinoma arising in pleomorphic adenomas.
Material and Methods
Case selection
A selected series of 27 cases of carcinoma arising in pleomorphic adenoma were retrieved from the files of two Oral Pathology Departments in Aleppo, and Al-Farabi Dental School [Table/Fig-1]. Normal tissue of the salivary gland, surrounding the tumour, was used as a control in the 27 cases of carcinoma which arose in pleomorphic adenoma (PA). The criteria proposed by Nagao et al., [10] for defining carcinoma ex-pleomorphic adenoma were used to select and reclassify our cases of carcinoma ex-pleomorphic adenoma.
Clinical data of 27 carcinomas ex-pleomorphic adenomas cases (CXPA)
| CXPA Cases | Age | Gender | Gland | Histological subtype | Metastasis to lymph nodes* |
|---|
| 1 | 77 | F | Parotid | Adenocarcinoma | Yes |
| 2 | 28 | M | Parotid | Adenocarcinoma | No |
| 3 | 78 | M | Submandibular | Undifferentiated | Yes |
| 4 | 45 | M | Parotid | Undifferentiated | Yes |
| 5 | 76 | F | Parotid | Undifferentiated | No |
| 6 | 82 | F | Parotid | Undifferentiated | No |
| 7 | 71 | M | Parotid | Adenocarcinoma | No |
| 8 | 67 | M | Submandibular | Undifferentiated | Yes |
| 9 | 63 | M | Submandibular | Undifferentiated | Yes |
| 10 | 55 | M | Submandibular | Undifferentiated | Yes |
| 11 | 73 | M | Parotid | Undifferentiated | Yes |
| 12 | 71 | M | Parotid | Undifferentiated | No |
| 13 | 64 | M | Parotid | Undifferentiated | Yes |
| 14 | 60 | F | Parotid | Undifferentiated | Yes |
| 15 | 49 | F | Submandibular | Undifferentiated | No |
| 16 | 39 | F | Parotid | Undifferentiated | Yes |
| 17 | 56 | M | Parotid | Undifferentiated | No |
| 18 | 45 | F | Parotid | Undifferentiated | Yes |
| 19 | 57 | M | Parotid | Undifferentiated | Yes |
| 20 | 66 | F | Parotid | Undifferentiated | No |
| 21 | 86 | F | Submandibular | Undifferentiated | Yes |
| 22 | 17 | F | Parotid | Undifferentiated | No |
| 23 | 78 | M | Submandibular | Undifferentiated | Yes |
| 24 | 26 | M | Parotid | Undifferentiated | No |
| 25 | 31 | F | Parotid | Undifferentiated | No |
| 26 | 71 | M | Parotid | Undifferentiated | No |
| 27 | 71 | M | Parotid | Undifferentiated | No |
F: female M: male, * Metastasis to lymph nodes at the time of tumour resection
According to the World Health Organization histological clasification which was published in 2005, malignant changes in the PA include three different types: CXPA, carcinosarcoma, and metastasizing PA The inclusion criteria for carcinoma ex-pleomorphic adenoma compromised major gland primary lesions (parotid or submandibular), and the macroscopic features that suggested a malignant transformation in pleomorphic adenomas, included poorly defined and/or infiltrative tumour margins, the presence of foci of haemorrhage, and necrosis. Also, the co-existent benign and malignant elements were considered as well. Benign element can be a pleomorphic adenoma within the tumour mass, a biopsy proven history of a previous PA (pleomorphic adenoma) which had indicated that it was in the same location as that of the subsequent carcinoma. Malignant elements can be undifferentiated carcinoma, adenocarcinoma, and multiple patterns of differentiation, including undifferentiated or adenocarcinoma patterns.
Exclusion criteria for carcinoma ex-pleomorphic adenoma includes the other well recognized salivary carcinomas and those of uncertain type, included in the current WHO histological classification of tumours [11]. The immunohistochemical expression of antibodies against p16 was examined in the selected cases.
Microscopic slides stained with haematoxylin and eosin were reviewed by two pathologists to confirm the histopathological diagnosis and to reclassify the studied cases. Ethical approval was provided by research ethics committee (Ref: 09/1016).
Immunohistochemistry
Paraffin-embedded tumour samples stored in pathology laboratory files were used in this study. Serial 4-μm- sections were consecutively cut from all 27 specimens. The sections were deparaffinized in xylene and they were rehydrated by passing through graded alcohols. Sections were processed by using streptavidin-biotin-peroxidase method. Briefly, the endogenous peroxidase was blocked by 3 % hydrogen peroxidase for 5 min, followed by washing with TBS (Tris buffered saline). Nonspecific immunoreactivity was blocked by incubation with normal goat serum for 20 minutes. A purified mouse anti-human monoclonal antibody p16 (Pharmingen, San Diego) was diluted to 5ml in 10ml tris buffer saline (TSA), which contained 0.1 % bovine serum albumin, for 1 hour at room temperature. All sections were washed by TBS for 5 minutes. Sections were incubated with the biotinylated secondary antibody reagent for 30 minutes, followed by washing in TBS for 5 minutes. Slides were incubated with streptavidin and horseradish peroxidase for 30 minutes, followed by washing in tris buffered saline (TBS) for 5 minutes and they were incubated with a prepared chromogenic substrate solution (Diaminobenizidine) for 15 minutes. Sections were counterstained with 0.25 % methyl green in distilled water for 5 minutes. They were dehydrated and mounted in Depax. Squamous cell carcinoma was used as a positive control. Negative control was used only with substitution of the primary antibody with TBS. The staining pattern was classified according to the relative number of positive cells in the different epithelial layers of the specimens. A brown precipitate which was seen within the nucleus, confirmed the presence of protein. A total of five areas were chosen randomly from each of the tested slides and they were scored at high power magnification. The nuclear staining was observed exclusively in the nuclei of the test cells. None of the negative controls displayed brown staining in the test cells. The percentage of p16 positive nuclei was semiquantively assessed by two independent observers and they were scored as: negative (0) no expression of nuclear protein, (1) weak staining 0-25 % of the total cells showing positive staining in the nucleus, (2) moderate staining >25–75% of the total cells in the test area showing positive nuclear staining, (3) strong staining >75-100% cells showing positive nuclear staining.
Statistical Analysis
The statistical analysis included the use of descriptive statistics; and frequencies proportion. Also, statistical analyses, including Wilcoxon’s nonparametric tests (ordinal data), were performed on the data. All statistical tests were two-sided and p-values of less than 0.05 were considered to be statistically significant.
Results
p16 expression in an adjacent area of carcinoma ex–pleomorphic adenoma p16 expression of the nuclear staining was studied in an adjacent area of carcinoma ex-pleomorphic adenoma. p16 nuclear staining of duct cells showed strong positive nuclear staining in 23 (85%) cases out of 27 cases [Table/Fig-2], 3 (11.1%) showed moderate staining, and 1 (3.7%) showed weak staining. p16 nuclear staining of the acinar cells showed negative staining in 1 (3.7%) case out of 27 cases, 11 (40.7%) showed weak staining, and 15 (55.5%) showed moderate staining.
p16 expression shows strong staining in normal tissue surrounding carcinoma ex-pleomorphic adenoma
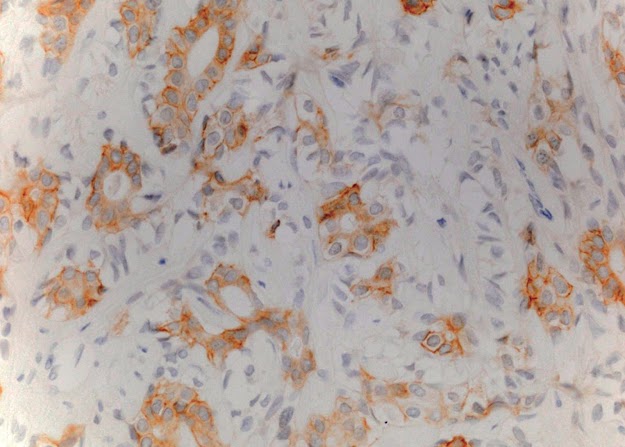
p16 expression in carcinoma ex–pleomorphic adenoma p16 expressed negative nuclear staining in 22 (81.4 %) cases out of 27 cases, and 5(18.5%) cases expressed modeate staining [Table/Fig-3]. There was a significant difference (Wilxocon test, p value < 0.001) between p16 expression in the nucleus in the duct cells of normal tissue surrounding the tumour and in the tumour area. 23 cases out of 27 cases showed strong positive staining which was detected in the duct cells (normal tissue) and no strong staining was noted in all cases in tumour duct cells.
p16 expression shows moderate staining in carcinoma ex-pleomorphic adenoma
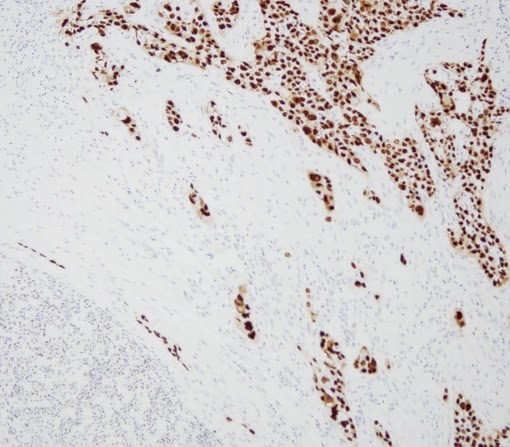
Discussion
This study focused on p16 pathway protein, to demonstrate the deregulation of p16 cell-cycle control in carcinoma ex-pleomorphic adenoma. Our results suggested that the p16 pathway was frequently deregulated in carcinoma ex-pleomorphic adenoma (p16 expressed a negative nuclear staining in 22 (81.4 %) cases out of 27 cases). Only few papers have been published on p16 expression in carcinoma ex-pleomorphic adenoma, because of rarity of this tumour as compared to others. Our results differed from those of others. Schache et al., [12] demonstrated the successful application of qMSP (quantitative methylation-specific real-time polymerase chain reaction) to a large series of historical (CXPA) samples and they reported on a panel of tumour suppressor gene, p16, with significant differences in their methylation profiles between benign and malignant variants of pleomorphic salivary adenoma. They concluded that qMSP analysis might be a useful clinical tool for differentiating between CXPA and its benign precursor. Patel et al., [13] reported that p16 was more likely to be expressed in the malignant components of CXPA than in the benign components of pleomorphic adenoma (positivity rates 69% versus 81%). Gong et al., [14] reported that p16 and nm23 genes may play important roles in different sides in salivary gland tumourigenesis and that the reduced expressions ofp16 and nm23 genes may contribute to the generation of malignant salivary gland tumours.
Zhu et al., [15] mentioned that the positive unit of p16 was higher in tumour group and cancer group of salivary gland than that in normal group of salivary acini (p < 0.01). The normal duct cell proliferation rate was higher in non tumour duct cells than in acinar cells, because 23 cases out of 27 cases showed a positively strong nuclear staining in normal duct cells but they did not show any strongly positive staining in the acinar cells. Zhu et al., [16] indicated that the current histogenic theory of salivary gland tumourigenesis considered the acinar cells as functionally mature cells and they suggested that the acinar cells were terminally differentiated and that they were incapable of further proliferation. Also, this theory considered that proliferation for the purpose of repair and regeneration was confined to stem cells residing exclusively amongst luminally located intercalated duct cells or to basally located excretory duct cells. Kim et al., [17] indicated that it was important to report the histological subtype of CXPA and to assess potential biomarkers such as p53, VEGF, c-erbB-2, c-kit, and glut-1 in diagnostic and therapeutic trials. Hashimoto et al., [18] indicated that S100P may play an important role in malignant transformation of ductal cells of PA, and that immunohistochemical staining for S100P would be a useful diagnostic marker for identifying the early phase of CXPA, in combination with androgen receptor, HER2, p53, and Ki-67. Tarakji et al., [19] showed that the nuclear P53 was expressed strongly in 6/29 (20.7%) pleomorphic salivary adenomas and in 10/27 (37%) carcinoma ex-pleomorphic adenomas and that there was no significant difference between p53 expression in pleomorphic adenomas (tumour duct cells) and in carcinoma arising in pleomorphic adenoma (p-value>.05).
The interpretation of the variations in the detection of p16 staining These differences may have resulted due to the following reasons: The use of different antibodies, different classifications e.g (0=negative staining, 1=low, 2= moderate, 3= strong or 0-3= negative and 4= positive or 0-2= negative and 3-4=positive or negative and positive staining), fixation times and concentrations of antibodies, and the sensitivity of the technique used.
The assessment of the positive or negative nuclear staining cells is controversial. Many authors have used different criteria and so, the results cannot be compared. In the present study, the rational of 75% break point may provide a more complete assessment of protein expression and a clearer understanding of the roles played by potential tumour markers in predicting outcome.
The immunostaining technique is used only in combination with another technique e.g (Polymerase Chain Reaction, Western Blotting) to detect and confirm existence of a mutation. Unfortunately, we did not use other techniques such as Polymerase Chain Reaction, Western Blotting to confirm the immunostaining results, for which further studies are recommended. Many studies used criteria such as negative, low, moderate, and strong staining.
Conclusion
The sample of carcinoma arising in pleomorphic adenoma cases is large (27 cases) as compared to others, though further research is required to increase the sample size, to determine the role of p16 in the pathogenesis of carcinoma arising in pleomorphic adenoma. p16 was altered in CXPA. Further research is oriented to extract DNA from the studied cases, to detect mutations as a probable main cause of inactivation and to identify other causes of inactivation such as methylation or loss of heterozygosity .