Biopsy is the diagnostic test of choice for oral potentially malignant disorders and malignant lesions.But since scalpel biopsy is an invasive procedure associated with potential morbidity,several adjunctive screening aids are used to assist clinicians with the detection of early cancerous changes [1]. Although oral exfoliative cytology is a simple non-invasive technique, the traditional exfoliative cytology methods show low sensitivity (i.e. a high proportion of false negatives), inadequate sampling, procedural errors, and the need for subjective interpretation of the findings [2]. Routinely shed oral epithelial cells can be detected in saliva and oral rinses, making cytologic and molecular analysis of this fluid attractive for oral cancer screening. In the present study epithelial cells collected via oral rinse method are subjected to routine cytologic analysis along with the conventional exfoliative cytology smear. The advantages of this method are increased patient comfort and the possibility of oral rinse collection by anyone, including the patient himself, without the requirement of health personnel or armamentarium, especially in resource challenged areas.
Material and Methods
Hundred and five subjects from the Department of Oral Medicine, A.B. Shetty Memorial Institute of Dental Sciences, Deralkatte, India with normal oral mucosa were selected for the study. The study protocol was approved by the Committee on Ethics of the Nitte University, Mangalore, Karnataka, India. Patients were informed with regard to the research objectives, methods, possible benefits and potential risks, and a written consent was obtained from all participants.
Careful examination of the oral cavity was followed by exfoliative cytology (oral rinse based technique followed by wooden spatula based cytology).
Techniques employed: Oral rinse technique was used to collect oral cells. Patient was asked to swish his/her mouth with water and expectorate. Then, the clinically normal buccal mucosa was rubbed on firmly by the patient themselves using their tongue for 30 seconds. While swishing phosphate buffered saline, pH -7.2 was used and patient was asked to expectorate into a sterile container. Once the sample was obtained, it was labelled and centrifuged at 1,000 rpm for 5 minutes. Supernatant fluid was discarded and smears were prepared from the cell plug. For exfoliative cytology, scrapings were obtained from clinically normal buccal mucosa using a standard moistened wooden spatula. Scrapings were smeared on labelled glass slides. All the slides thus prepared were immediately fixed in absolute alcohol and consequently stained with Papanicoloau stain.
All slides were assessed by 2 trained cytopathologists, who were not aware of the type of the technique from which the material was collected. For comparative analysis of both techniques three parameters were used: a) cell yield b) cellular dispersion and d) cellular clarity. Both the observers were asked to grade each parameter into Good /Average / Poor in a data sheet comprising a total of five parameters. These gradings were given a numerical value of 3, 2 and 1 respectively and the observers were referred to as O1 and O2. Inter-examiner agreement between cytopathologist 1 and cytopathologist 2 was good (kappa=0.715) for conventional and oral rinse based cytology, respectively.
Results
Among the 105 subjects, three and one samples were considered unsatisfactory on conventional cytology and oral rinse based cytology, respectively. In conventional cytology, samples were disregarded due to excessive clumping or scarcity of cells. In oral rinse based cytology one sample was rejected due to scarcity of cells [Table/Fig-1]. Depicts the parameter assessment scoring by both the observers (O1 and O2). Mann Whitney test (nonparametric) was used to calculate the test of significance (p ≤ 0.05.value) using minitab software for windows(release 12). Statistical analysis of cell yield, cellular dispersion and cellular clarity in both the smears using wooden spatula and oral rinse showed a statistically significant difference between the two methods [Table/Fig-2].
Assessment of three parameters by two observers (O1 ,O2) in slides prepared from both the techniques
| Parameters | Wooden spatula | Oral Rinse |
|---|
| 09 | O1 | O2 | O1 | O2 |
| Scoring by the observers |
| Cell yield | 260 | 243 | 282 | 266 |
| Cellular dispersion | 248 | 260 | 262 | 276 |
| Cellular clarity | 247 | 268 | 292 | 271 |
Statistical analysis of the parameters using Mann Whitney Test
| Parameters | Number of Samples | Median | Point Estimate | 95.0 Percent Confidence Interval | w–value | p–value |
|---|
| Cell yield Wooden spatula | 101 | 5.0000 | 0.0000 | (-0.9999,0.0001) | W = 8704.0 | 0.0001(adjusted for ties) significant |
| Cell yield Oral rinse | 101 | 6.0000 |
| Cellular dispersion Wooden spatula | 101 | 5.0000 | -0.0000 | (-1.0001,0.0001) | W = 9175.0 | 0.0052(adjusted for ties) significant |
| Cellular dispersion Oral rinse | 101 | 5.0000 |
| Cellular clarity Wooden spatula | 101 | 5.0000 | -1.0000 | (-1.0001,0.0000) | W = 8438.0 | <0.0001(adjusted for ties) significant |
| Cellular clarity Oral rinse | | 6.0000 |
Discussion
Although conventional cytology was used for evaluating oral lesions as far back as 1963, [3] it has not been widely adopted and has fallen into disrepute in most centers because of poor sensitivity and specificity for identifying dysplasia and malignancy. However, it should not be forgotten that whilst histological assessment is the accepted method of diagnosis, the histopathological interpretation of dysplastic lesions can also be prone to subjectivity [4].
During the 1980’s, a cytobrush was introduced for cervical smears in gynecological lesions. The adaptation of the cytobrush for oral cancer diagnosis helped revive major interest in oral cytology. Since then, various studies have been published describing different diagnostic techniques that have improved the sensitivity and specificity of conventional oral cytology [5]. A similar study was undertaken to compare the efficiency of Cytobrush with that of wooden tongue spatula. The Cytobrush was found to be significantly more efficient than the wooden spatula, in terms of both cell yield (p less than .005) and cell dispersion (p less than .005). The study showed that the Cytobrush was an effective instrument for use in exfoliative cytology of normal oral mucosa [6].
Further the oral brush biopsy came into light, with computer-assisted analysis and was simple to perform, non-invasive, and had the potential to overcome many of the obstacles that had hindered early detection of early stage cancers and dysplasia [7]. Oral CDx (OraCDx Laboratories, Suffern, NY) is a computer-assisted method for the analysis of cellular samples collected by using a patented brush. This technique was designed to evaluate any oral epithelial abnormality without an obvious etiology for dysplasia or cancer [8]. However, some authors were of the opinion that Oral cancer is a poor man’s disease and the methods of diagnosis should be cheap and accurate [8]. Thus the authors have no doubt in their minds to say that the oral CDX brush biopsy is irrelevant for oral cancer detection in developing countries [9]. Several markers assessed within oral exfoliative cytology samples have shown promise ,although each in isolation have limitations [4].
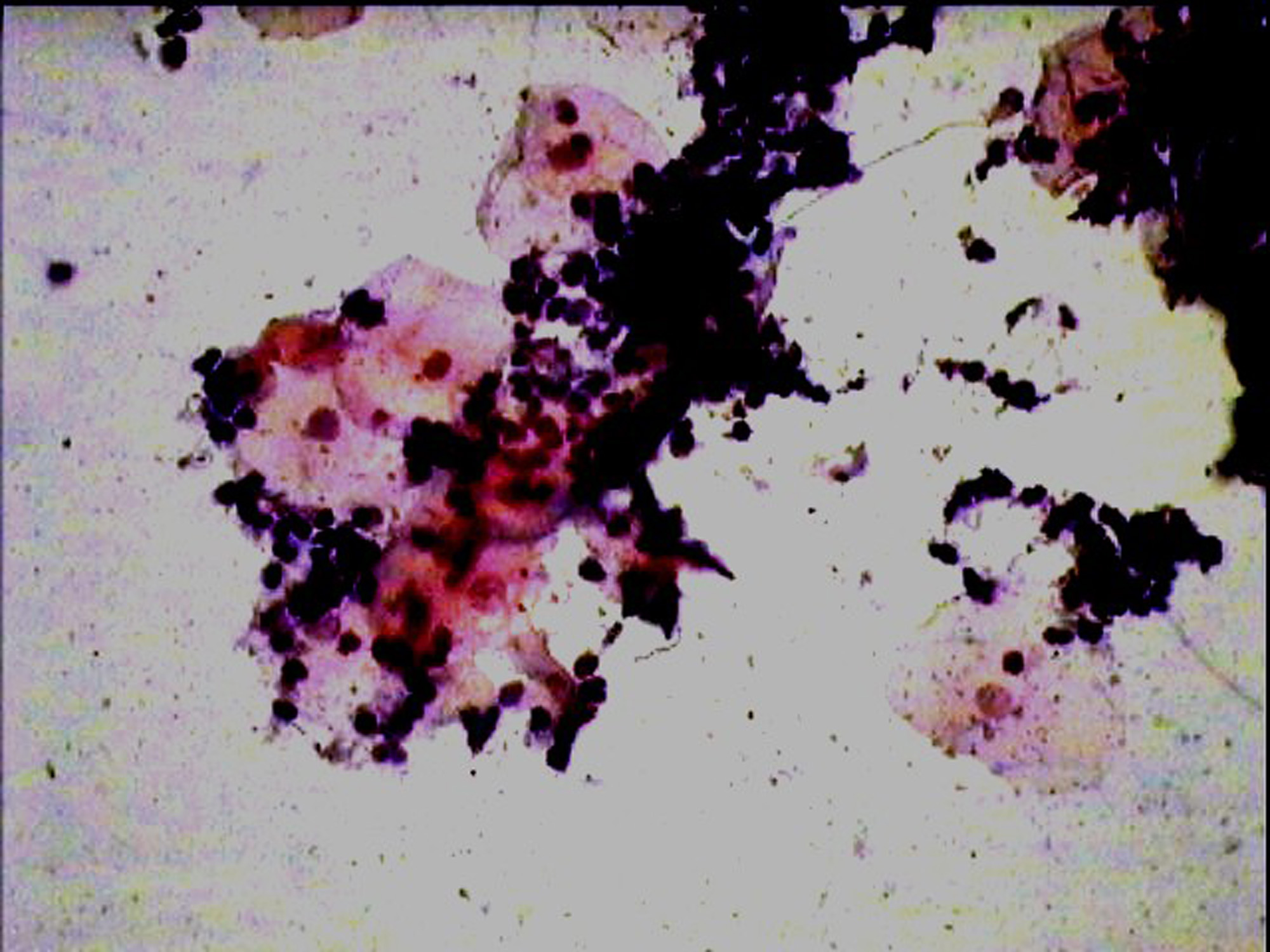
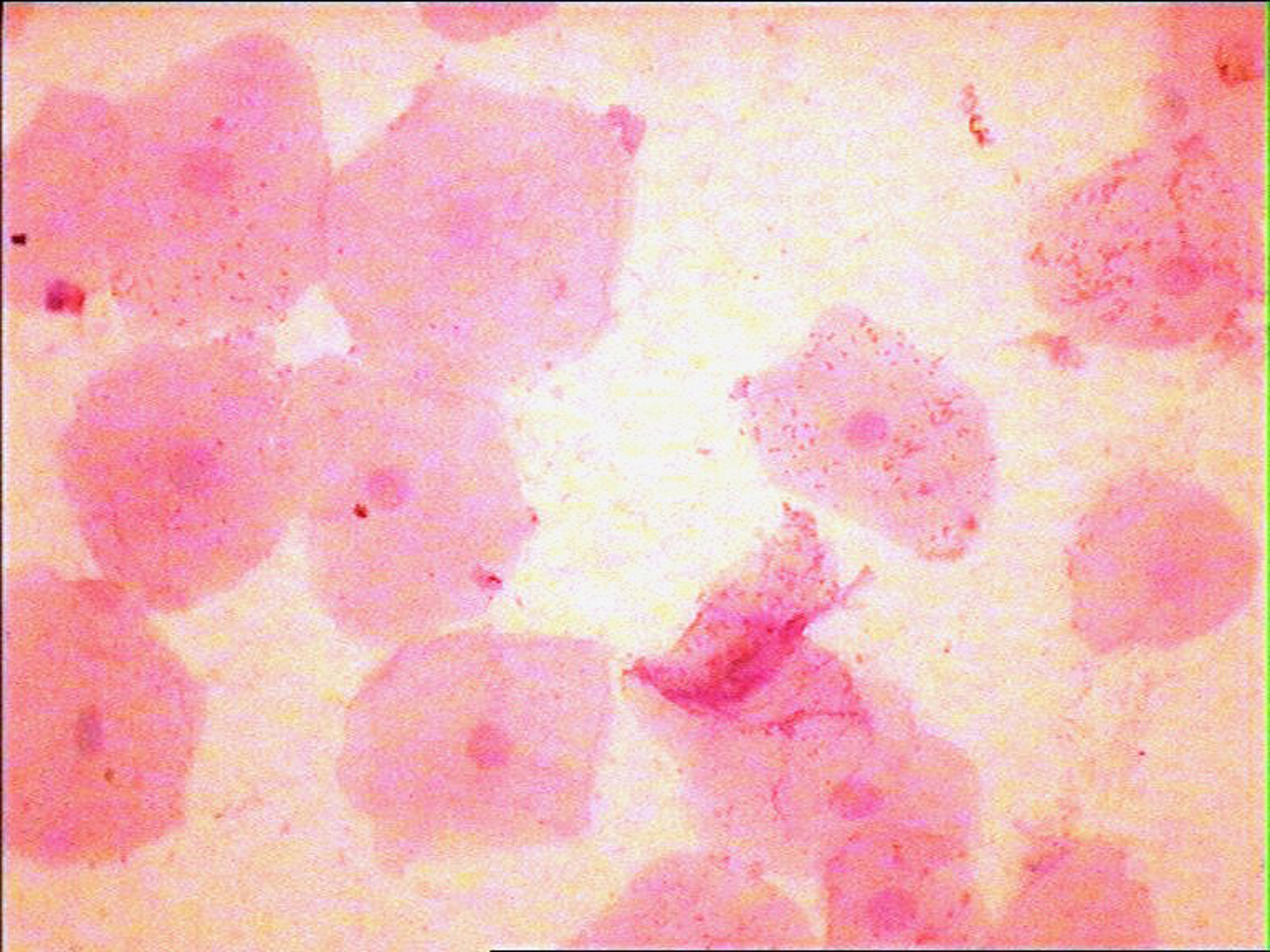
The development of a reliable test in the detection and follow-up of malignant lesions, which is painfree, simple,less time consuming, cost effective and reproducible, remains the objective. The oral rinse technique is one such method which meets most of these criteria. Added advantages of this method are increased patient comfort, ease in sample collection especially in resource challenged areas and epidemiologic studies. Also, overlapping of cells and debris [Table/Fig-3] which is a common problem with the conventional exfoliative cytology can be considerably reduced in oral rinse smears [Table/Fig-4], thus providing a clearer background for better visualization of cells in a debris free smear. But the procedure requires well trained staff for proper processing of the sample, otherwise it can result in poor quality of the smears.
Photomicrograph of a smear showing overlapping of cells in a smear prepared with wooden spatula X400(Pap)
Photomicrograph of a oral rinse smear showing cells in a clear background X400(Pap)
The use of liquid based cytology can offer improved and repeatable preparations than conventional cytology, and reduce the false negative results. The liquid based cytology reduces the problems related to sampling and fixation and presents a better cytological morphology. Both sensitivity and specificity are better in liquid based cytology than in conventional cytology [10]. As liquid-based cytology method allows for the preparations of more than one slide per sample collected, there always will be enough material for other techniques besides Papanicolaou stain, such as PAS and Methamine Silver. Finally, the material preserved in the solution has a long storage life; therefore remaining may be available for additional analyses like immunostaining [10].
There are several studies based on oral liquid-based cytology. All these studies are based on liquid based cytology using a brush to collect the cells, in contrast to the present study which uses oral rinse directly to collect cells. Hence, the present study too can be considered as a liquid based cytologic method. A similar study compared specimen adequacy and diagnostic agreement between liquid-based preparations using a brush and conventional smears in oral lesions, and also tested the viability of immunocytochemical assay in liquid-based preparations from oral carcinoma lesions. They inferred that both the smears were diagnostically reliable; and that the liquid-based method showed an overall improvement on sample preservation, specimen adequacy, visualization of cell morphology and reproducibility [10]. A case-control study was developed to evaluate the sensitivity, specificity, and concordance between conventional cytology and liquid-based cytology which comprised of patients with primary Oral Squamous Cellular Carcinoma (OSCC) (case group) and normal buccal mucosa (control group). When compared, conventional cytology and liquid-based cytology showed a high sensitivity and reasonable specificity [11].
Oral rinse technique has been in use for long, mainly for microbiological purposes, especially to analyse oral candidal colonization [12]. Recently the same technique was utilized to detect oral squamous cell carcinoma [13]. Epidemiological studies conducted earlier concluded that both a 10-ml oral-rinse sample and 2-ml whole-saliva sample provide sufficient DNA quantity and better quality DNA for genetic epidemiological studies than do the commonly used buccal swab and brush techniques. Since the present technique utilized PBS for collection of cells, remaining cells can be stored and used later for immunohistochemistry and DNA studies [14].
The present study was conducted as a preliminary study to assess the quality of the oral rinse based smears. Further studies with larger sample size and including potentially malignant disorders and oral cancer is being executed. It revealed that oral rinse based cytology resulted in improved quality of cell yield, cellular dispersion and clarity among other advantages.
Conclusion
Oral rinse based cytology may be used as a convenient alternative to traditional exfoliative cytology in normal oral mucosa.