Growing Teratoma Syndrome of Ovary: Avoiding A Misdiagnosis of Tumour Recurrence
Ananya Panda1, Devasenathipapathy Kandasamy2, Chandrashekhara SH3, Manisha Jana4
1Senior Resident, Department of Radiodiagnosis,Dr BR Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences,Ansari Nagar, New Delhi-110029, India.
2Assistant Professor, Department of Radiodiagnosis,Dr BR Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110029, India.
3Assistant Professor, Department of Radiodiagnosis,Dr BR Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110029, India.
4Assistant Professor, Department of Radiodiagnosis,Dr BR Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110029, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Devasenathipathy Kandasamy, Assistant Professor, Department of Radiodiagnosis, BRAIRCH, All India Institute of Medical Sciences (AIIMS), Ansari nagar, New delhi, 110029, India.
Phone: 91-9873391531, 91-9013283425,
E-mail: devammc@yahoo.co.in
Growing teratoma syndrome is characterized by conversion of immature gonadal germ cell tumour to a mature form, along with increase in size of the lesions. Being more frequently described in testicular germ cell tumours, growing teratoma syndrome in an ovarian tumour is uncommon. The exact cause of conversion and growth is unknown and is hypothesized to be induced by chemotherapy. On imaging, the diagnosis is suggested by the appearance of mature elements such as fat or calcium within these lesions and it is confirmed by normal serum markers despite the increase in its size. Knowledge of this entity is important, to avoid misdiagnosis as disease progression and continuing chemotherapy, as these lesions are refractory to chemotherapy. Though surgery is curative, they can also be safely followed-up in a majority of cases, with a favourable long term prognosis.
Growing teratoma syndrome, Ovarian immature teratoma, Bleomycin Etopside Cisplastin (BEP) chemotherapy
Case Report
A 29–year–old, married female first presented to our hospital in July 2011, with gradual abdominal distension and dull aching pain in lower abdomen, of 6 months duration. There was no loss of weight or appetite. Past medical history and surgical history were not significant. There was no family history of similar complaints. The patient was para one and had been having regular menses. Per abdomen examination revealed a distended abdomen and a vague ill-defined mass in right lower abdomen. Per vaginal examination was normal. In view of the clinical symptoms and examination findings, a contrast-enhanced CT (CECT) scan of chest, abdomen and pelvis was done.
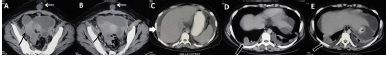
CECT scan of chest and abdomen revealed a 6 x 5.5 x 4 cms, solid-cystic, ill-defined right adnexal mass with peritoneal deposits, ascites, bilateral pleural effusion, parietal deposit in infraumbilical anterior abdominal wall and a metastatic nodule in lung [Table/Fig-1].There was no loco-regional lymphadenopathy. In view of the appearance of the adnexal mass, a provisional diagnosis of an ovarian neoplasm was thought of and considering the young age of the patient, the first differential diagnosis made was a germ cell tumour, most likely an immature teratoma or a dysgerminoma. The possibility of an epithelial ovarian neoplasm such as a mucinous or a serous cystadenocarcinoma was also considered in differential diagnosis. However, since these epithelial cell tumours are seen in older age groups, beyond fourth decade, they were kept lower down in the list of differentials. The patient’s serum alpha foeto-protein (AFP) and human chorionic gonadotrophin (hCG) levels were also elevated, thus favouring possibility of a germ-cell tumour. Ultrasound guided-biopsy confirmed the diagnosis of an immature germ cell tumour of ovary.
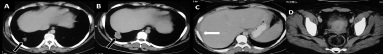
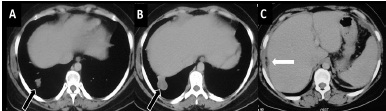
She underwent right salpingo-oophorectomy and excision of anterior abdominal wall lesion, followed by 3 cycles of bleomycin, etoposide and cisplatin (BEP) chemotherapy. Repeat CECT scan done in October 2011 revealed residual disease with a liver surface deposit and a metastatic pulmonary nodule [Table/Fig-2].Three months later, follow-up scan done in Jan 2012 showed nearly same size of liver surface deposit and pulmonary lesions, suggestive of stable disease [Table/Fig-3]. The serum tumour markers studied in Jan 2012 were found to be within normal limits and the lady became clinically asymptomatic.
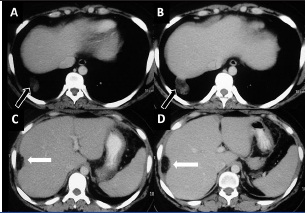
The latest scan done in October 2012 showed an increase in size of liver surface deposit and pulmonary lesion, raising a possibility of disease progression. Thus, a possible diagnosis of tumour recurrence was considered. But simultaneously, there was also fatty conversion within these lesions [Table/Fig-4]. This fatty attenuation within the growing masses held the key to the diagnosis and a hence, final diagnosis of “growing teratoma syndrome (GTS)” was made. A subsequent normal serum AFP level (3.72 ng/ml) obtained, confirmed the diagnosis of GTS.
Since the lady is currently asymptomatic, she has been kept on clinical and biochemical follow-ups.
A-E: Baseline preoperative contrast-enhanced CT: Axial images A to E show a solid cystic mass in right hemipelvis (black arrow in A, B) with anterior abdominal wall deposit (white arrow in A, B). Additional metastatic deposits noted in perihepatic region (white thick arrow in C) and in right lower lobe (block arrow in D, E). Also note ascites with bilateral pleural effusions (C-E)
A-D: Post-operative post 3 cycles chemotherapy. Axial CECT images show residual pulmonary lesion (block arrows in A, B), residual perihepatic deposit causing scalloping of liver surface (arrow in C) and minimal ascites. There is no residual mass in right adnexal region and abdominal wall deposit has also resolved (D). These findings indicate partial response
A-C: Post 6 cycles chemotherapy. Axial CECT images show nearly same size of both the pulmonary lesion (block arrow in A,B) and the perihepatic lesion (arrow in C). There is residual ascites. Since the lesions had not significantly changed in size and no new lesion was noted, it was labelled as stable disease. Also note that the perihepatic lesion has become partially fatty attenuating
A-D: Follow-up scan 9 months later: Axial CECT images show increase in size of both pulmonary (block arrow A, B) and perihepatic (white arrow C, D) lesions. All lesions show fatty attenuation and the perihepatic lesion is completely fat-attenuating despite significant increase in size. Features suggestive of growing teratoma syndrome
Discussion
GTS is an unusual syndrome seen post-treatment in cases of nonseminomatous germ cell tumour, with a reported incidence of 1.9 to 7.6% [1-2]. First formally named and described by Logothetis et al in 1982, it comprises of enlarging chemotherapy-refractory masses containing mature teratomatous elements [1]. While a growing teratoma syndrome after treatment of non-seminomatous testicular germ cell tumours has been widely described [3], its appearance secondary to malignant germ cell tumours of ovary is very rare and less than twenty case reports or series have been described till date. A similar phenomenon has been separately described in ovarian neoplasms, termed as “chemotherapeutic retroconversion”. This comprises of distant metastases with benign mature elements and it can be considered to be synonymous with growing teratoma syndrome [4]. However, in GTS, the masses have the ability to grow, which is lacking in chemotherapeutic retroconversion [2].
The exact cause of GTS is unknown and various hypotheses include (A) a chemotherapy-induced transformation of malignant to benign cells or (B) a natural selection of benign cells, with malignant cells being killed by chemotherapy or (C) a spontaneous evolution of malignant totipotent germ cell into benign tissues [2,5].
The diagnosis can be made, based on a combination of imaging evidences of increasing size of masses containing fat, calcification or cystic changes in a patient with a history of a germ cell tumour with normal tumour markers, during or after completion of chemotherapy [6]. Recently, absence of activity on FDG-PET has been shown to be useful in confirming the benign nature of these lesions [7]. The metastatic masses can occur anywhere in the pelvis, retroperitoneum, liver, lungs or mediastinum and onset of their appearance after completion of treatment is variable. The lesions often grow rapidly in size and they can lead to mass effect or compressive symptoms, which may require surgery.
Since these lesions are refractory to chemotherapy, surgery is the only curative treatment for them. A surgical excision is usually done to alleviate compressive symptoms or to prevent the very rare possibility of a sarcomatous transformation within these lesions [3]. A long term prognosis is generally considered to be favourable [8] and resumption of menstruation and successful pregnancies have also been reported in two cases [9-10].
Conclusion
Growing teratoma syndrome of an ovarian non-seminomatous germ cell neoplasm is an unusual entity which is characterized by multiple enlarging masses composed of mature elements. Despite the increase in their sizes, neither should these be mistaken for signs of disease progression nor should chemotherapy be given, as serum tumour marker levels remain normal and lesions are chemorefractory. Though the final diagnosis depends on the histologic confirmation of mature elements, it is important for a radiologist to be aware of this entity as appearance of intralesional fat, calcification or cystic changes on CT scan, can suggest the proper diagnosis and avoid a mismanagement.
[1]. CJ Logothetis, ML Samuels, A Trindade, The growing teratoma syndromeCancer 1982 50:1629-35. [Google Scholar]
[2]. V Gorbatiy, PE Spiess, LL Pisters, The growing teratoma syndrome: Current review of the literatureIndian J Urol 2009 25:186-89. [Google Scholar]
[3]. DM Panicek, GC Toner, RT Heelan, Nonseminomatous germ cell tumors: enlarging masses despite chemotherapyRadiology 1990 175:499-502. [Google Scholar]
[4]. H Amsalem, M Nadjari, D Prus, Growing teratoma syndrome vs chemotherapeutic retroconversion: case report and review of the literatureGynecol Oncol 2004 92:357-60. [Google Scholar]
[5]. JG Lorigan, F Eftekhari, CL David, The growing teratoma syndrome: an unusual manifestation of treated, nonseminomatous germ cell tumors of the testis.AJR Am J Roentgen 1988 151:325-29. [Google Scholar]
[6]. HB Tongaonkar, VH Deshmane, Dalal Growing teratoma syndromeJ Surg Oncol 1994 55:56-60. [Google Scholar]
[7]. S Kikawa, Y Todo, S Minobe, Growing teratoma syndrome of the ovary: a case report with FDG-PET findings.J Obstet Gynaecol Res 2011 37:926-32. [Google Scholar]
[8]. R Hariprasad, L Kumar, D Janga, Growing teratoma syndrome of ovaryInt J Clin Oncol 2008 13:83-7. [Google Scholar]
[9]. AR Sengar, JN Kulkarni, Growing teratoma syndrome in a post laparoscopic excision of ovarian immature teratomaJ Gynecol Oncol 2010 21:129-31. [Google Scholar]
[10]. G Tzortzatos, A Sioutas, K Schedvins, Successful pregnancy after treatment for ovarian malignant teratoma with growing teratoma syndromeFertil. Steril 2009 931:36-3. [Google Scholar]