Extragastrointestinal Stromal Tumour of The Abdominal Wall - A Case Report
A. Sathish Selva Kumar1, R Padmini2, G Veena3, N Murugesan4
1 Assistant Professor, Department of Pathology, Affiliated to ESIC-Medical College & PGIMSR, K.K.Nagar, Chennai-78, India.
2 Senior Resident, Department of Pathology, Affiliated to ESIC-Medical College & PGIMSR, K.K.Nagar, Chennai-78, India.
3 Senior Resident, Department of Pathology, Affiliated to ESIC-Medical College & PGIMSR, K.K.Nagar, Chennai-78, India.
4 CMO/Senior Resident, Department of Surgery, Affiliated to ESIC-Medical College & PGIMSR, K.K.Nagar, Chennai-78, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. A. Sathish Selva Kumar, F-4, Shiv Apartments, Kalaiamagalnagar, Ekkattuthangal, Chennai-600032, Tamil Nadu, India.
Phone: 09884858501,
E-mail: sathishpath17@gmail.com
Stromal tumours occurring in areas other than the GastroIntestinal Tract (GIT) are known as Extra GastroIntestinal Stromal Tumours (EGISTs). They usually arise in the mesentery, omentum or retroperitoneum, while EGISTs which occur in the abdominal wall are very rare. Both gastrointestinal stromal tumours (GISTs) and EGISTs are histologically and immunophenotypically similar. We are reporting a case of EGIST, which occurred in the anterior abdominal wall in a twenty five-year-old female patient. The tumour was present in the right loin and imaging studies suggested that it was a desmoid tumour. It was surgically excised by doing an abdominal wall mesh repair. The histological examinations revealed a tumour with spindle cell morphology, with <2 mitoses per 50 High Power Field (HPF) and no necrosis, with tumour free margins. Immunohistochemistry was strongly positive for CD117 and Smooth Muscle Actin (SMA), while it was negative for β-catenin and S100. The patient is well post operatively and is on close follow up. EGISTs should be considered in the differential diagnosis of mesenchymal tumours which occur in the abdominal wall, inspite of their rarity, as the high risk patients may need Imatinib chemotherapy.
CD117, β-catenin, Imatinib
Case Report
A twenty-five-year-old female presented with a mass, which gradually increased in size, (of three months duration), which was associated with vague pain. She had undergone a Lower Segment Caesarean Section (LSCS) four years ago.
Examination and investigation
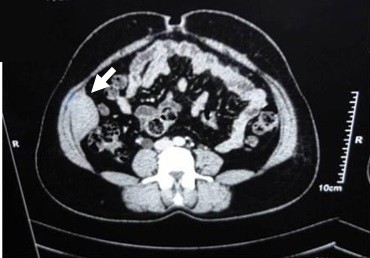
On examination, a firm mass of sixe 6x5cm was felt in the right lumbar region. The mass was non tender, non pulsatile, it extended upto the right iliac fossa and moved with respiration. Contrast Enhanced Computed Tomography (CECT) showed a well-defined heterogeneous mass of size 6x5x3cm, arising within the mid portion of the right transverse abdominis muscle and partly adherent to the internal oblique and peritoneum. Other system examinations were within normal limits. Ultrasound guided Fine Needle Aspiration Cytology (FNAC) report of the mass was inconclusive. A pre-operative diagnosis of a desmoid tumour was considered [Table/Fig-1].
CECT scan abdomen, axial section shows (white arrow) mass of size 6 x 5.5 x 3 cm arising within the mid portion of the right transverse abdominal muscle and partly adherent to the internal oblique and peritoneum
Exploratory laparotomy
Intraoperatively, the tumour attachments from the abdominal wall muscles and peritoneum were released and they were resected with margin clearance. The defect was repaired by using a polypropylene (6cmx11cm) mesh. The post-operative course was uneventful.
Histopathological examination
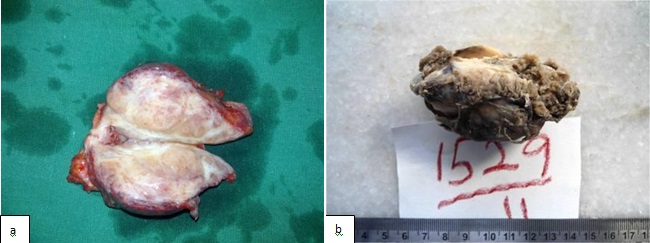
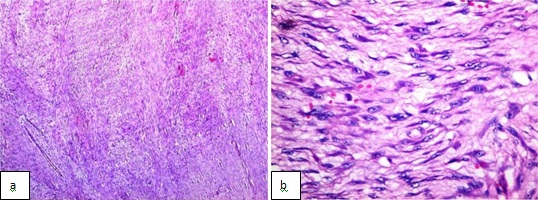
Gross examination showed, a fairly circumscribed, grey white, solid mass measuring 5.8x 4.5x 4 cm, with focal areas of congestion [Table/Fig-2]. Microscopy, revealed a tumour which was arranged in vague, short fascicles. The cells were spindle shaped with eosinophilic cytoplasm and centrally placed oval nucleus. Less than 2 mitoses per 50 High Power Field (HPF) were found. No necrosis was seen. The stroma was made of fine strips of collagen which were interrupted by a delicate vasculature. All resected margins were free of the tumour [Table/Fig-3].
Gross: a) immediately after surgery b) after formalin fixation. Cut section show a fairly circumscribed solid grey white mass measuring 5.8x4.5x4cm with focal areas of congestion
Hematoxylin and eosin stained sections a) show short spindle shaped cells forming short ill defined fascicles (X40) b) show the short fusiform nature of the individual cells and infrequent mitoses (<2/50HPF) (X400)
ImmunoHistoChemistry (IHC)
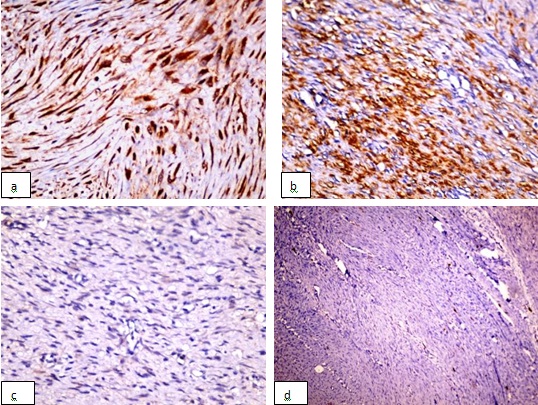
The tumour showed a strong cytoplasmic positivity for CD117. The Smooth Muscle Actin (SMA) showed a positive staining, while β-catenin and S100 showed a negative staining, which ruled out the possibility of a desmoid tumour [Table/Fig-4]. A histopathological diagnosis of an EGIST was made. The patient is on close follow-up after twenty three months and is free from any local recurrence or distant metastasis.
Immunohistochemistry evaluation: a) CD117 (c-kit) showing a strong cytoplasmic positivity (X100) b) smooth muscle actin (SMA) showing strong positivity (X100) c) β-catenin show negative staining (X40) d) S100 marker show negative staining (X40)
Discussion
Gastrointestinal Stromal Tumours (GISTs) are mesenchymal tumours that usually arise from the gut wall [1]. They express the phenotype of interstitial cells of Cajal or related stem cell-like precursors with frequent c-KIT mutations [1,2]. GISTs most commonly arise from the stomach, followed by jejunum and ileum, colon, rectum and oesophagus [3]. About 5% to 7% of GISTs arise from the omentum, mesentery and retroperitoneum and they are referred to as Extragastrointestinal Stromal Tumours (EGISTs) [4]. They have similar biological behaviours and principles [1]. EGISTs commonly occur in adult age group, with around 80% of them occurring in the omentum and the rest occurring in the retroperitoneum, while those which occur in abdominal wall are extremely rare [5]. A majority of the cases are considered as metastases or a result of loss of contact with a primary intra-abdominal tumour [1,6]. Our case did not reveal any primary source and the histomorphology, along with immunohistochemical (IHC) analysis, helped in diagnosing it to be as a primary EGIST of the abdominal wall.
Seventy percent of EGISTs have a spindle cell morphology, while 20% have an epithelioid cell morphology and the remaining 10% have both [1,7]. The present case demonstrated a spindle cell morphology with short fusiform shaped cells, spindle to oval shaped nuclei and with light staining eosinophilic cytoplasm, which were arranged in short ill-defined vague fascicles. The stroma was made of fine collagen strips which were interspersed by a thin vasculature [1]. The cells which are found in leiomyomas and leiomyosarcomas are usually elongated in nature [Table/Fig-3]. Extensive hyalinization, especially that which occurs around the vessels can resemble hemangiopericytomas in 20% of the cases [1].
EGISTs have immunohistochemical patterns which are similar to those of GISTs and CD117 (c-KIT) is the most sensitive and specific marker which can be used to confirm their diagnosis [8]. CD117 shows a strong and diffuse, cytoplasmic, membranous or dot-like paranuclear (golgi-like) positivity [1]. Our case had a strong cytoplasmic positivity. Desmoid tumours can be differentiated, on the basis of CD117 negativity and beta catenin positivity [4a,c]. Melanomas, angiosarcomas, seminomas and mast cell tumours should be ruled out due to their positive c-KIT staining [1]. Hirota et al., in 1998, described the presence of gain of a functional mutation in the proto-oncogene, c-KIT, a type III tyrosine kinase receptor which is located on chromosome 4 (4q11-q12), which leads to the development of GISTs [2]. c-KIT mutations which occur in 90% to 95% of GISTs, cause ligand-independent phosphorylation and constitutional activation, leading to a tumourigenic activity [2,9]. Other markers that are useful are, CD34 and SMA, while desmin, vimentin and keratin show variable positivity [1]. About 5% of GISTs have a mutation of Platelet Derived Growth Factor A (PDGFRA) [1].
No clear guidelines exist currently, to predict the outcome of EGISTs, because of the rarity of these tumours [1,7]. However, the risk stratification criteria which was put forth by the National Institute of Health for GIST in 2001, based on tumour size and mitotic activity can be used in for EGISTs [1,7]. The effects of different extragastrointestinal sites of occurrence and the varied mutational status on the risk assessment is still far from clear [1]. Cases with high cellularity (which are noted as areas with frequent nuclear overlapping), a mitotic activity of >2 mitoses/50 High Power Field, and any amount of necrosis, are considered to be high risk cases that frequently metastasized [1]. However, no correlation was noted between the tumour morphology, immunophenotypic profile and the outcome [1]. Imatinib mesylate (Gleevac), an ATP analogue binds to KIT and inhibits the oncogenic signaling pathways. It is usually preferred in metastatic and unresectable high risk cases [1,10].
Conclusion
The relative increased frequency of fibromatoses in the abdominal wall of a female patient with a history of trauma, both iatrogenic and mechanical, should not deter one from considering EGIST as a differential diagnosis. Histopathological and immunohistochemical examinations,especially,the strong positivity of CD117 and a β-catenin negative staining, will delineate the diagnosis. Disease risk stratification as per consensus guidelines for GISTs can be followed in EGISTs, with surgery and a long-time follow-up in low risk cases and a targeted therapy in high risk cases. However, more data regarding treatment, prognosis, disease characterisation and evaluation of targeted therapy outcome is required.
[1]. Weiss SW, Goldblum JR, Soft tissue tumours: Fibromatoses 2008 Fifth editionPhiladelphiaMosby Elsevier:565-81. [Google Scholar]
[2]. Hirota S, Isozaki K, Moriyama Y, Gain of function mutations of c-KIT in human gastrointestinal stromal tumorsScience 1998 :279-577. [Google Scholar]
[3]. West R, Coreless C, Chen X, Rubin B, Subraamanian S, Montgomery K, The novel marker, DOG 1 is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation statusAm J Pathol 2004 165:107-13. [Google Scholar]
[4]. Emory TS, Sobin LH, Lukes L, Prognosis of gastrointestinal smooth-muscle (stromal) tumorsAm J Surg Pathol 1999 23:82 [Google Scholar]
[5]. Thalheimer A, Meyer D, Gattenlöhner S, Timmermann W, Thiede A, Gastrointestinal stromal tumor of the abdominal wall. An unusual localization of a rare tumorChirurg 2004 75:708-12. [Google Scholar]
[6]. Agaimy A, Wünsch P, Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentationLangenbecks Arch Surg 2006 391(4):322-9. [Google Scholar]
[7]. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Diagnosis of gastrointestinal stromal tumors: a consensus approachInt J Surg Pathol 2002 10:81-9. [Google Scholar]
[8]. Loiy Alkhatiba, Omar Albtoushc, Nesreen Batainehb, Gharaibeha Kamal, Ismail Matalkab, Yasuharu Tokudad, Extragastrointestinal Stromal Tumor (EGIST) in the abdominal wall: Case report and literature reviewInternational Journal of Surgery Case Reports 2011 2:253-55. [Google Scholar]
[9]. Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, CD-117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34Mod Pathol 1998 11:728 [Google Scholar]
[10]. Heinrisch MC, Corless CL, Demetri GD, Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumorJ Clin Oncol 2002 21:4342 [Google Scholar]