Solitary Fibrous Tumour (SFT) is an unusual spindle cell tumour that usually occurs in the pleura, but has recently also been reported to be extra-pleural in origin. A renal presentation is very rare. Upto 90% of the tumours have benign characteristics. It is difficult to differentiate it from renal cell carcinoma by using imaging techniques. A definitive diagnosis can be made by doing a detailed pathological examination, which includes immunohistochemistry. We are reporting a case of a large solitary fibrous tumour of the kidney which here occurred in a 70-years-old male. Histological examination of the resected specimen confirmed the diagnosis, by revealing strongly positive reactions of the neoplastic cells for CD34, bcl-2, vimentin and negativity for Epithelial Membrane Antigen (EMA), Smooth Muscle Actin (SMA), S-100 protein and Ki-67. The patient suffered a cardiac arrest and died on the seventh day after his surgery.
Extra-pleural, Immuno-histochemistry
Case Report
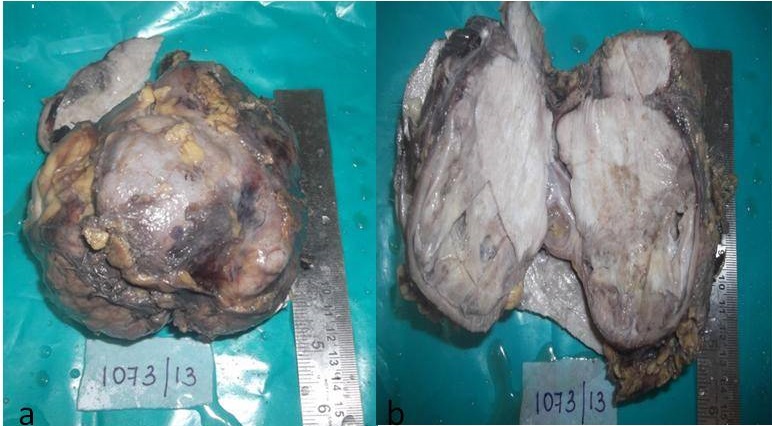
A 70-year-old male patient presented with right-sided loin pain which he had since one month. He was a known case of hypertension and chronic obstructive pulmonary disease since six years. His clinical examination revealed a large, bimanually palpable mass in the right lumbar region. An ultrasonographic examination revealed a large, hypoechoic mass lesion which occupied the right kidney. CT scan revealed a large, heterogenous enhancing mass lesion in upper pole, which involved the entire kidney. Superoanteriorly, the mass abutted the inferior surface of liver and gall bladder, posteriorly into the para-renal space, in close approximation to psoas muscle. The mass measured 106 x 88mm, with involvement of right renal vein and it possibly extended into the inferior vena cava. Right renal Doppler showed a large exophytic mass which was seen in upper, mid and lower poles of right kidney posteriorly, which measured 129 × 82 × 72mm. A radiological diagnosis of renal cell carcinoma which involved the entire kidney with the pelvicalyceal tissue, was made. The patient underwent a right radical nephrectomy and the kidney was subjected to a histopathological examination. Macroscopically, the specimen measured 13 × 10 × 8cm [Table/Fig-1a]. Cut surface showed an ill-circumscribed, irregularly infiltrating, homogenous, firm grey white tumour which involved the entire kidney, with areas of cystic degeneration [Table/Fig-1b]. Microscopy revealed a tumour which was arranged in solid sheets and fascicles, which diffusely infiltrated and destroyed the normal renal parenchyma [Table/Fig-2a]. Prominent hyalinized collagenous tissue was seen [Table/Fig-2b]. The cells were from round to oval to spindly and were arranged around vascular spaces. A scanty, preserved, pelvicalyceal lining mucosa was the only remnant of normal renal tissue which was left. Mitoses were occasional. Few tumour giant cells and minimal necrosis were seen. A histopathological impression of an aggressive vascular tumour was made. Immunohistochemical markers were used to confirm the histopathological diagnosis [Table/Fig-3a, 3b, 4a, 4b and 5]. The neoplastic cells were diffusely positive for CD34, bcl-2, vimentin. They showed weak, focal positivity for CD99 and were negative for epithelial membrane antigen, smooth muscle actin, S-100 protein. Less than 5% of the cells showed nuclear positivity with Ki-67. A confirmatory diagnosis of a solitary, fibrous renal tumour was made.
(a) Right nephrectomy specimen - enlarged, with intact, bosselated, smooth external surface. (b) Well-circumscribed, homogeneous, grey white tumour compressing the normal renal parenchyma and extending into the perinephric fat
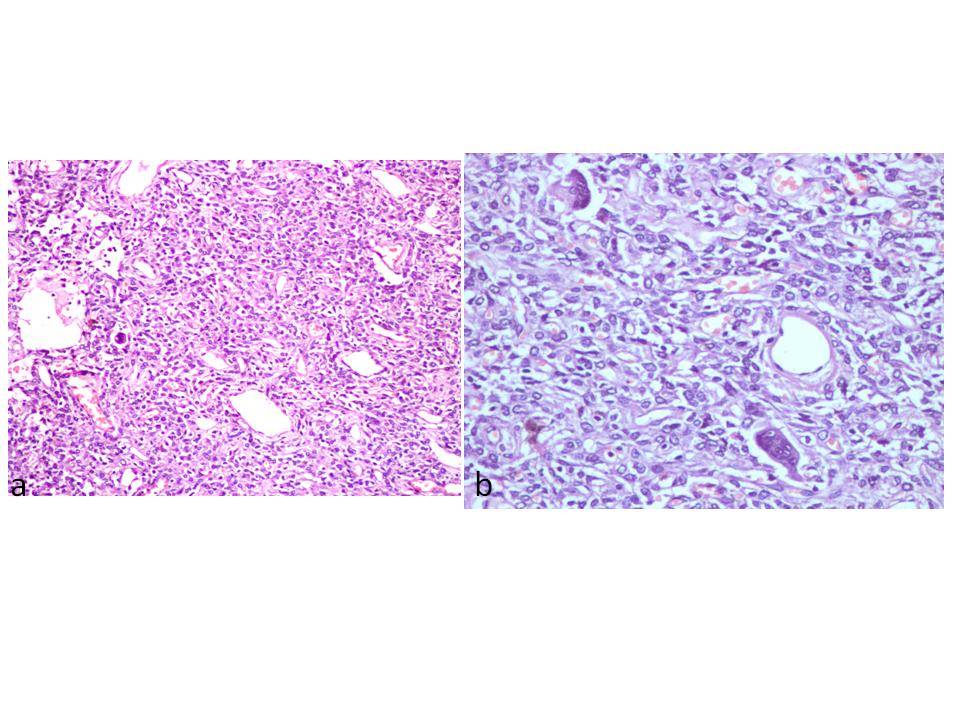
(a) Photomicrograph of the tumour showing spindle cells arranged in fascicles in a haemangiopericytoma- like pattern (H & E, x 100). (b) Giant cells and collagen in the tumour (H & E, x 200)
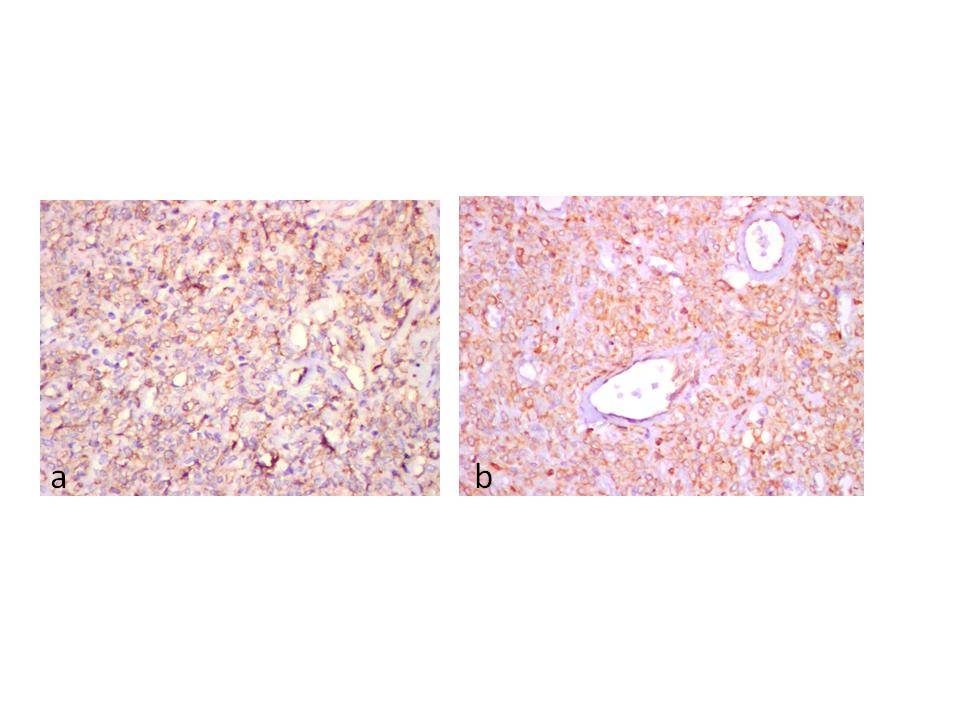
(a) Diffuse and strong positive cytoplasmic staining of tumour cells for CD34 ( IHC, x 200). (b) Diffuse membrane staining of tumour cells for bcl-2 (IHC, x200)
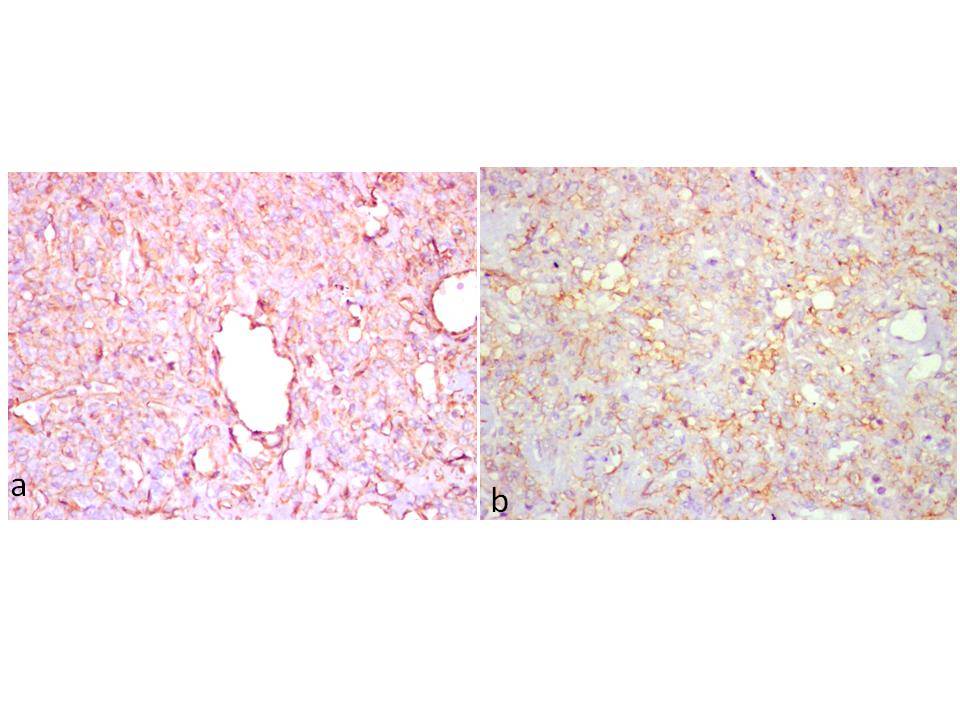
(a) Diffuse cytoplasmic positivity of tumour cells for vimentin (IHC, x200). (b). Focal membrane positivity of tumour cells for CD99 ( IHC, x 200)
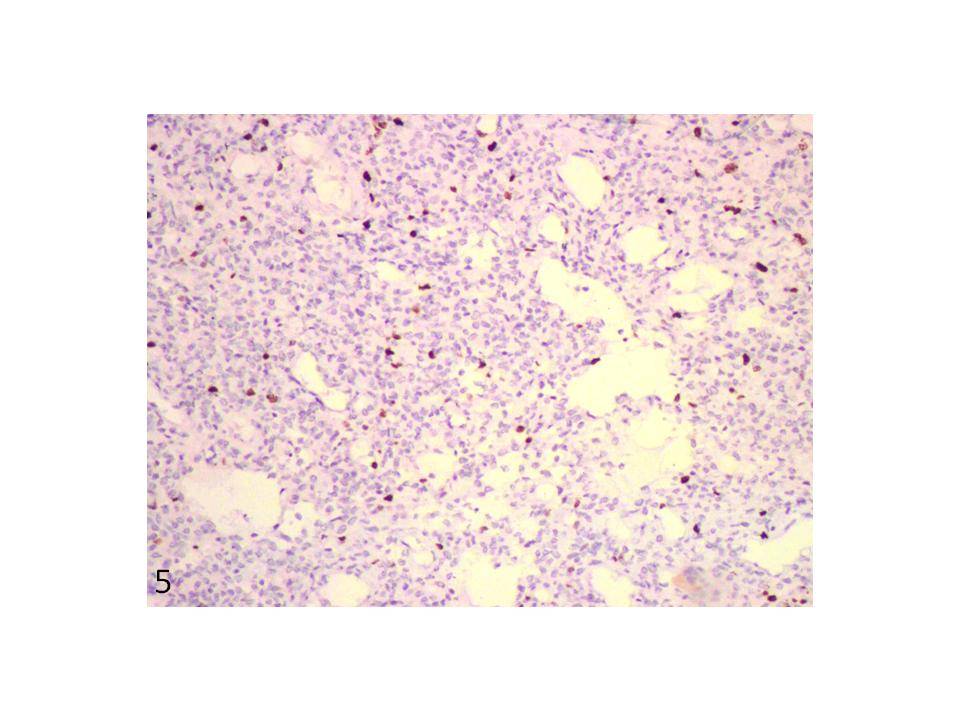
Tumour cells show < 5% nuclear staining with Ki-67 (IHC, x200).
Discussion
Solitary Fibrous Tumours (SFTs) are rare mesenchymal neoplasms which are considered as variants of haemangiopericytomas. They mostly originate from the pleura. Other extra-pleural locations include the orbit, paranasal sinuses, nasal cavity, upper respiratory tract, lung, major salivary glands, breasts, meninges, liver and urogenital organs [1]. Historically, haemangiopericytoma and SFTs are the two uncommon tumours that have been regarded as distinct entities, but their overlapping clinical and histologic features suggest that they may represent ends of a continuum [2]. Renal solitary fibrous tumours are rare, with only 47 cases having been previously reported worldwide to date [3]. Indeed, in 1942, Stout and Murray introduced the concept that haemangiopericytomas originated from the pericytes of blood vessels [4]. But nowadays, they are classified as solitary fibrous tumours to a large part, because they are clearly fibroblastic than pericytic in nature. According to WHO classification, a haemangiopericytoma is described as a lesion that consists of SFT and related conditions, which include giant cell angiofibromas and lipomatous haemangiopericytomas [4]. The SFT, as a well-established neoplasm, was first reported by Klemperer and Rabin in 1931, as a tumour of the pleura [5]. In the kidney, SFTs arise from the capsule, renal pelvis or hilar fatty tissue [6]. More than 50% SFTs have occurred in patients who were older than 40 years [4]. Age range from 28-85 years. The male to female ratio appears to be almost equal (1:1.5) [4]. Grossly, renal SFTs which have been reported in literature ranged in size from 2 to 25 cm. (mean 8.75 cm). Most of them were well-circumscribed or pseudo encapsulated, lobulated, rubbery or firm masses with a homogeneous, grey or tan white, whorled cut surface [4]. An occasional case had areas of cystic changes, haemorrhage or foci of necrosis.
Various radiologic findings of renal SFT can be seen. They may appear as hypo or heterogeneous echoic masses on ultrasound or as well-circumscribed smooth lobulated solid enhancing masses on CT [7]. Large masses mimic malignancies and our case clinically masqueraded as a malignant growth.
Roughly 10-15% of all SFTs are malignant. The criteria for malignancy include their atypical locations, increased mitotic activity (more than 4 mitoses per 10 high power fields), necrosis, haemorrhage, infiltrative margins and p53 expression [6]. Nevertheless, a histopathological prediction of an aggressive behaviour of the tumour is difficult [6]. Clinical behaviour cannot be adequately predicted on histopathological basis, with benign-appearing tumours exhibiting aggressive behaviour and vice versa [1]. SFTs must be differentiated from benign and malignant spindle cell tumours of the kidney [5]. It is particularly difficult to differentiate them from haemangiopericytomas which have similar histological findings and CD34 positivity. Haemangiopericytomas macroscopically contain haemorrhagic areas. As compared to SFTs, haemangiopericytomas have less cellular diversity and stromal hyalinization. While CD34 positivity is weak in haemangiopericytomas, it is diffuse and strong in SFTs. Other tumours from which SFTs can be hardly distinguished are fibromas, inflammatory myofibroblastic tumours, angiolipomas, leiomyomas, schwannomas, neurofibromas, haemangiomas, angiosarcomas, synovial sarcomas and sarcomatoid renal cell carcinomas, as all of the above show haemangiopericytomatous patterns [8]. A long term follow-up is mandatory, as metastases can still occur after more than 15 years.
Immunohistochemistry sets the final diagnosis. A panel of immunostains is typically necessary, to exclude other spindle cell proliferations that may simulate the histologic image of SFTs . The profile of the tumour is highly characteristic. SFTs reveal diffuse positivity for CD34, which is now considered as a characteristic marker of SFT [8]. 70% of SFTs express CD99 and bcl-2 and only 20-35% are variably positive for Epithelial Membrane Antigen (EMA) and smooth muscle actin. In general, SFTs show negative reactivity for cytokeratin, alpha-SMA, S-100 protein and c-kit [9]. These contribute to the differential diagnosis of SFTs from other spindle cell tumours. Neurofibromas may be reactive for bcl-2, CD34 and they typically express S-100 protein, CD 56/57 unlike SFTs. Synovial sarcomas show bcl-2 reactivity, but they lack CD34. Spindle cell lipomas are CD34 and S-100 positive, but they lack histologic features of SFTs. Myxoid variants of SFTs can be potentially mistaken for other myxoid lesions such as low grade fibromyxoid sarcomas, myxoid liposarcomas, myxoid malignant peripheral nerve sheath tumours. Myxoid liposarcomas and low grade fibromyxoid sarcomas are CD34 negative [8,10]. Our case showed diffuse positivity for CD34, bcl-2, vimentin, weak focal positivity for CD99 and negativity for EMA, SMA, S-100 protein. Ki-67, as a proliferative marker, showed <5% positivity, which was consistent with the immuno-histochemical profile for SFTs.
An adequate resection is the mainstay of therapy of renal SFTs, which was done in our case. Though in our case, the patient had other comorbid factors contributory to death; the definitive diagnosis was arrived at, due to complete immunohistochemical workup which was done.
Conclusion
SFTs are unusual mesenchymal tumours with a good prognosis. Their confirmatory diagnosis can be made on the basis of combined morphological, microscopic and immunohistochemical workups. They may be confused with malignant tumours. Pathologists must be careful in this respect and patients with such tumours need to be on long-term follow-ups. This case has been presented for its rarity and variable mode of prognosis.
[1]. Sfoungaristos S, Papatheodorou M, Kavouras A, Perimenis P, Solitary Fibrous Tumour of the Kidney with Massive Retroperitoneal Recurrence. A Case PresentationPrague Medical Report 2012 113:246-50. [Google Scholar]
[2]. Serrano MF, Humphrey PA, Adult Renal Neoplasms, Surgical diseases of the KidneyThe Washington Manual of Surgical Pathology2nd EditionEditors: Humphrey Peter, Dehner LP, Pfeifer JD Lippincoat Williams and Wilkins [Google Scholar]
[3]. Nazih K, Raja K, Mohammad S, Jad D, Ibrahim K, Jessica A, Solitary fibrous tumours of the kidneys: Presentation, evaluation, and treatmentUrol IntDOI 10.1159/000354394, Aug 29, 2013 [Google Scholar]
[4]. Znati K, Chbani L, Fatemi HE, Harmouch T, Kamaoui I, Tazi F, Solitary Fibrous Tumour Of the Kidney: A Case Report and Review of the LiteratureRev Urol 2007 9:36-40. [Google Scholar]
[5]. Sasaki H, Kurihara T, Katsuoka Y, Nakano T, Yoshioka M, Miyano S, Distant Metastasis From Benign Solitary Fibrous Tumour Of The KidneyCase Rep Nephrol Urol 2013 3(1):1-8. [Google Scholar]
[6]. Demirtas A, Sabur V, Akgun H, Akmsal EC, Demirci D, Solitary Fibrous Tumour of the Kidney: A Case ReportCase Reports in Urology Volume 2013 Article ID 147496, http://dx.doi.org/10.1155/2013/147496,4 pages [Google Scholar]
[7]. Park SB, Park YS, Kim JK, Kim MH, Oh YT, Kim KA, Solitary Fibrous Tumor of the Genitourinary TractAmerican Journal of Roentgenology 2011 196(2):W132-37. [Google Scholar]
[8]. Wick MR, Hornick JL, Immunohistology of Soft Tissue and Osseus NeoplasmsDiagnostic Immunohistochemistry Theranostic and Genomic applications3rd editionSaunders ElsevierEditor David J Dabbs [Google Scholar]
[9]. Makris A, Tabaza R, Brehmer B, Lindemann-Docter K, Wildberger J, Jakse G, Solitary fibrous tumor of the kidney: a case reportThe Canadian Journal of Urology 2009 16(5):4854-56. [Google Scholar]
[10]. Naveen HN, Nelivigi GN, Venkatesh GK, Suriraju V, A case of solitary fibrous tumour of the kidneyUrol Ann 2011 3(3):158-60. [Google Scholar]