Several studies have performed on biological samples to detect genotoxic effects in humans in connection to their jobs or environment. It is of increasing concern that professional personnel usually expose to anti-neoplastic drugs during preparation and administration [1]. It has been reported that multiple professional workers from clinical, pathology laboratories, farmers and welders are exposing to carcinogenic compounds [2–4]. However, the identification of substances capable of inducing mutations has become an important procedure in safety assessment. Urine mutagenicity is recognized as a marker of internal dose for detecting recent exposure to mutagens in bio monitoring studies of populations exposed to environmental and/or workplace-generated complex mixtures [5–9].
Thereby, in vitro studies have essential roles for the evaluation of urinary mutagenicity test by using Salmonella typhimurium indicator strains to monitor populations of occupationally or environmentally exposed to genotoxic compounds [10].
The purpose of this study was determine the anti-mutagenic effects of Vitamin E on oncology and non-oncology hospital nurses by Ames assay. Thus, in the constant quest for new therapeutic natural products have been tested for their antioxidant and antigenotoxic effects [11,12].
In animal model, Vitamin E has been shown to inhibit completely peroxidative injury by restoring renal tissue antioxidants and glutathione redox balance of hyperoxaluria-induced renal injury [13], also it has been shown that Vitamin E ameliorates both the cardiac damage and carcinogenicity of the quinones adriamycin and daunomycin, which are mutagenic and carcinogenic [14].
In the course of these studies, we specifically evaluated the anti-mutagenic effects of Vitamin-E in hospital nurses that is associated with anti-neoplastic drugs exposure.
Material and Methods
Study population
The study included 138 female (non-pregnant) nurses working in oncology and non-oncology hospitals located at Tabriz/Iran, between September 2011 to May 2012 and their duration of employment was (range 1–28 years). The first group consisted of 69 nurses working in a specialized oncology hospital, who had regular contact with anti-neoplastic drugs daily (preparation of solutions and syringes for infusion, administering of anti-neoplastic drugs and handling of body fluids of patients undergoing chemotherapy). The most frequently used chemotherapeutic drugs were doxorubicin, bleomycin, vinblastine, dacarbazine, methotrexate, fluorouracil, prednisone, epirubicin, irinotecan, leucoverin, prednisone, 6-mercaptopurine, procarbazine, lomustine, cisplatin, etoposide, 6-thioguanine and Cyclophosphamide. The handling time varied from 1 to 6 h/day.
The second control group 69 nurses were selected from non-oncology general hospital with no history of occupational exposure to anti-neoplastic agents.
The exclusion and inclusion criteria of research population and selection criteria of study persons were based on a questionnaire. All subjects were asked to complete a face-to-face questionnaire, which included standard demographic data (age, gender) as well as medical (exposure to X-rays, vaccinations, medication), lifestyle (smoking, coffee, alcohol, diet) and occupational questions (working hours per day, years of exposure, use of protective measures, etc.). It was assured that the exposed nurses and the controls did not statistically differ from each other except for anti-neoplastic drugs exposure. It was also ensured that the exposed and the control subjects had not been taking any medicines, nor had they been exposed to any kind of radiation for 12 months before sample collection. According to the ethical committee of Tabriz university of medical sciences, all subjects involved in the study received detailed information concerning the aims of the research study, available online: http://www.irct.ir, concern to WHO [15].
Reagents
All chemicals used in this study were of analytical grade and obtained from (Merck co, Germany). Vitamin-E (dL-alpha-tocopheryl acetate) pearl 200IU purchased from (Zahravi pharmaceutical company, Tabriz, Iran).
Collection of urine samples
After obtaining satisfactory and questionnaire forms from all the nurses, the primary morning urine samples (100 ml) all volunteers from the both groups were collected and transferred to laboratory in capped sterile beakers and labeled candidate nurses names. Then they received orally 200mg of Vitamin E daily for two weeks and at the end of 14th day second urine sample (100 ml) were also delivered to laboratory. The urine samples from the nurse volunteers were centrifuged at 3000g for 5 minutes and samples were stored at -20°C until required for extraction.
Urine samples screening
In order to verify the presence of chemotherapeutic drugs in urine samples [Table/Fig-5], all samples were screened by thin layer chromatography and in few samples as a result of Ames assay ratio ≥2, trace amount of chemotherapeutic drugs was performed [4,16].
Preparation of urine extracts
Extraction columns were filled with 1g from Amberlite XAD-2 resins between two layers of cotton, the resins were washed and activated with Milli-Q water and methanol for two times, then 100ml of urine samples of every volunteer passed from columns through vacuum. The column resins were rinsed with Milli-Q water again for elimination trace amount of interaction factor such as histidine then resins were dried with vacuum aspiration. The resin absorbed substances were washed with eluted solvent with equal mixture of methanol and Acetonitrile. Then these elusion solvents were evaporated with Nitrogen gas. The residue was dissolved in DMSO and refrigerated in −80°C until required for use [17,18].
S-9mix preparation
Male wistar rats weighed 250g (n=4) were selected and the homogenate of S-9mix fraction was prepared. The liver was immediately removed and kept in 1.15% sterilized KCL solution while working under strile conditions at 4°C. The known amount of liver tissue was minced and mixed with (1.15% KCL 3ml/mg). The mixure was homogenized and centrifuged at 9000g for at -20°C for 10 minutes using strile centrifuge vials. Aliquot the supernatant to strile cryo vials. The supernatants were sterilized by using the 0.2μ filter. This supernatant contain P450 microsomal sterile enzymes were stored frozen at −80°C for until use [19,20].
Media preparation
Glucose minimum agar content 10% glucose, Top agar content 0.5mM Biotin-Histidine, nutrient broth were prepared according to Mortelmans & Ziger [20] and were stored frozen for until use.
Ames assay
The samples of concentrated urine were assayed for mutagenicity with the Ames test as described by Mortelmans and Zeiger [20], by using S typhimorium tester strains in overnight cultures of TA100 S typhimorium tester strains with and without 10% S-9 mix fraction. For this strain, the spontaneous background number of the revertant was approximately 75-200 of colonies, TA100 S typhimorium tester strains with and without the 10% S-9mix fraction. In this study, Sodium azide (2.5μg/plate) was used as a positive control without using the S-9mix and the 2-aminoanthracene (0.5μg/plate) as a positive control with the S-9mix. DMSO and distilled water were considered as negative controls.
Statistical Analysis
The mean and Standard Deviation (SD) were analyzed for each Parameter. End point means (Mean Ames assay ratio) between oncology hospital and non-oncology hospital nurses were analyzed using Student’s t-test and Anova one way. Multiple linear regression analysis was assessed between end points of independent groups. All calculations were performed by using Graph Pad Prism 5 Software and SPSS. Anti-mutagenic effects of Vitamin E (exposure and age) was determined by using Student’s t-test and Anova one way. The level of significance considered was (p< 0.05).
Results and Observations
The effect of occupational exposure to chemotherapeutic drugs on the levels of carcinogenic compounds in exposed nurses and non exposed control subjects were assessed by the Ames assay. [Table/Fig-1] shows the results of specific characteristics of controls reversion colony counts.
Specific of positive and negative controls reversion colony counts
| Controls value of Salmonella typhimorium tester strain TA100 | μg or μl /Plate | -S-9Mix | +S-9Mix |
|---|
| 2-Amino antheracene | 0.5 | - | 2000 |
| DMSO | 50 | 220 | 250 |
| NaN3(Sodium aside) | 2.5 | 1500 | - |
| D.W(Diluted water) | 50 | 180 | 240 |
| Spontaneous value | - | 200 | 220 |
[Table/Fig-2] represents the distribution of subjects with respect to age, years of exposure and duration of handling chemotherapeutic agents per day. The two groups studied had similar demographic characteristics. The mean age of the exposed group was 36±7, ranging from 21 to 60 years, and that of controls was 36±7.3, ranging from 21 to 60 years. The extent of mutagenic activity in urine extracts evaluated by Ames assay with respect to biography, work history and ages of all the study subjects are listed in [Table/Fig-3 and 4]. [Table/Fig-3] shows the results of urine mutagenic activity before Vitamin E consumption in exposed and non exposed nurses. Also the results are explained in the [Table/Fig-5], indicates that oncology hospital nurses having higher mutagenic activity in urines as compare to the control group. It has been shown that before Vitamin E consumption approximately 25% of oncology hospital nurses excrete carcinogenic compounds in their urine. The mutagenic activity was increased by increasing the age 2.3±0.56, the observed value for non-exposed control was 1.25±0.52, p=0.11. Similarly the effect of Vitamin E among exposed and non exposed nurses is represented in [Table/Fig-4], as it is shown, the mutagenic activity was increased by increasing the age, so above the age of 36 the observed value was 2.3±0.94 and observed value in case of control was 1.19±0.59. [Table/Fig-5] shows the mutagenic activity in nursing group of exposed oncology nurses before and after Vitamin E consumption in the absence and presence of S-9 metabolic activation. Before Vitamin E approximately 25% urine extract was infected with mutagenic compounds (carcinogenic compounds were present in 12 urine extracts), while in the Vitamin E treatment significantly decreases of mutagenic compounds. However, in the corresponding non exposed group, mutagenic compounds were not observed. [Table/Fig-6] shows the anti-mutagenic effects of Vitamin E in the absence of S-9 mix among exposed oncology hospital nurses with respect to Ages, Years of exposure , exposure (hours) per day. Before Vitamin E the mutagenic activity was observed by increasing the age, so above the age of 36 the observed value was 2.3 ± 0.56, while Vitamin E treatment significantly decreases of mutagenic compounds and the observed value was 1.66 ± 0.35, p=0.000. However, the observed values below the age of 36 were (1.63 ±0.48 and 1.20 ± 0.62, p=0.001) respectively. Similarly, mutagenic activity among nurses who had been working in oncology hospital for more than 10.5 years (1.71 ± 0.50) versus Vitamin E treated nurses (1.30 ± 0.63, p=0.35). Those who worked for less than 10.5 years the observed data was not significant (1.72 ± 0.46 versus 1.11± 0.68, p=0.00001). Also Similar significant effects were observed regarding to the working hours per day. According to the figure 2, the regression line states anti-mutagenic effects of Vitamin E, who worked for less than 7.5 hours per day and among oncology exposed nurses Vitamin E treatment had been significant effects in reduction of mutagenic activity [Table/Fig-7].
Demographic characteristics of non exposed (control) and exposed nurses involved in the study.
| Parameters | Non-exposed nurses (n = 66) | Exposed nurses (n =66) | |
|---|
| Age (years) (Mean ± SD) | 36±7.3 | 36±7 | p = 0.28 |
| Years of exposure (Mean ± SD) | 11.13±7 | 10.46±7 | p = 0.33 |
| Per day exposure (hours) (Mean ± SD) | 7.78±1.77 | 7.18±0.76 | p = 0.14 |
t-test, p<0.05 significant for between groups
The results of urine mutagenic activity before Vitamin E consumption (Ames assay Ratio, without 10% microsomal S-9mix system) between exposed and non exposed nurses
| Parameters | Exposed Nurses Ratio | Non-Exposed Nurses Ratio |
|---|
| Age (years) |
| ≥36 | n=35 | 2.3±0.56 | n=35 | 1.25±0.52 p = 0.11 |
| <36 | n=31 | 1.63 ±0.48 | n=31 | 1.10±0.50 p = 0.06 |
| Years of exposure |
| ≥10.5 | n=34 | 1.71 ± 0.50 | n=34 | 1.20±0.54 p = 0.09 |
| <10.5 | n=32 | 1.72 ± 0.46 | n=32 | 1.14±0.49 p = 0.07 |
| Per day exposure (hours) exposure |
| ≥7.5 | n=31 | 1.77±0.0.45 | n=31 | 1.29±0.46 p = 0.07 |
| <7.5 | n=35 | 1.68±0.58 | n=35 | 1.09±0.55 p = 0.12 |
t-test, p<0.05 significant for between groups
The results of urine mutagenic activity after Vitamin E consumption (Ames assay Ratio, with10% microsomal S-9mix system) between exposed and non exposed nurses.
| Parameters | Exposed Nurses Ratio | Non Exposed Nurses Ratio |
|---|
| Age (years) |
| ≥36 | n=35 | 2.3±0.94 | n=35 | 1.19±0.59 p = 0.09 |
| <36 | n=31 | 1.87±0.72 | n=31 | 1.09±0.54 p = 0.09 |
| Years of exposure |
| ≥10.5 | n=34 | 2.22±0.83 | n=34 | 1.06±0.32 p = 0.06 |
| <10.5 | n=32 | 2.09±0.94 | n=32 | 1.12±0.56 p = 0.06 |
| Per day exposure (hours) exposure |
| ≥7.5 | n=31 | 2.4±1.1 | n=31 | 1.26±0.61 p = 0.056 |
| <7.5 | n=35 | 1.94±069 | n=35 | 1.01±0.37 p = 0.06 |
t-test, p<0.05 significant for between groups
The results of urine mutagenic activity before Vitamin E consumption (Ames assay Ratio, without 10% microsomal S-9mix system) between exposed nurses and non exposed nurses.
p<0.05 (Difference between exposed and non exposed nurses; student’s t-test)
a t-test, p= 0.09, n=35, b t-test, p = 0.09, n=31, c t-test, p= 0.06, n=34, d t-test, p=0.06, n=32, e t-test, p = 0.056, n=31, f t-test, p = 0.06, n=35
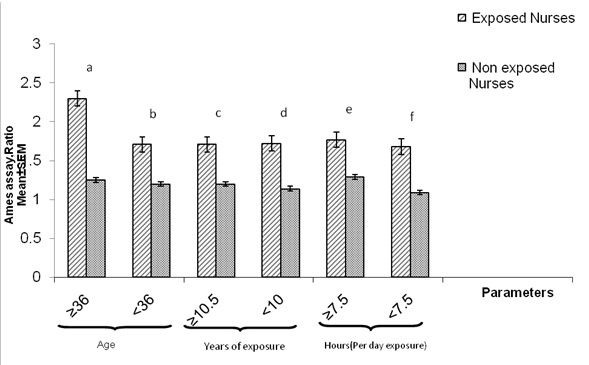
Anti-mutagenicity effects of Vitamin-E (Ames assay Ratio, without S-9mix) on (n=66) women oncology hospital nurses Paired t-test, p<0.05 significant importance
| Parameters | Exposed Nurses Ratio | Non Exposed Nurses Ratio |
|---|
| Age (years) |
| ≥36 | n=35 | 2.3 ± 0.56 | n=35 | 1.66 ± 0.35 P = 0.000 |
| <36 | n=31 | 1.63 ±0.48 | n=31 | 1.20 ± 0.62 P = 0.001 |
| Years of exposure |
| ≥10.5 | n=34 | 1.71 ± 0.50 | n=34 | 1.30 ± 0.63 P = 0.001 |
| <10.5 | n=32 | 1.72 ± 0.46 | n=32 | 1.11 ± 0.68 P = 0.00001 |
| Per day exposure (hours) exposure |
| ≥7.5 | n=31 | 1.77±0.0.45 | n=31 | 1.17±0.64 P = 0.00 |
| <7.5 | n=35 | 1.68±0.58 | n=35 | 1.24±0.66 P = 0.001 |
Regression line before and after 200mg/day Vitamin E consumption in oncology exposed nurses. n=34, work history ≥10.5 years, Paired t-test, p=0.001
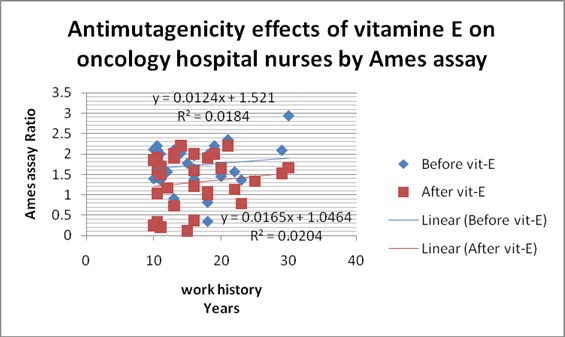
Discussion
The present study provides first evidence that Vitamin E supplementation significantly diminishes the mutagenic effects of anti-neoplastic drugs among nursing personnel. Chemotherapy agents with low therapeutic index having major role for chemical control of cancer and on this light, they induce mutagenic effects on normal cells. Studies have investigated the mutagenic potential of anti-neoplastic agents among the medical personnel chronically handling these drugs and it has been found that the excretion of mutagenic compounds in urine extracts are significantly higher than the control [21]. A study by Kosgeroglu, coworkers claimed that with regard to nursing self-protection, when the information and administration scores decrease and increase in the working period, may incur risk exposure to chemotherapeutic drugs [22]. So nursing and healthcare personnel, who admix, administer or dispose of anti-neoplastic drugs or body waste products from patients being treated with such drugs are at great risk of exposure to these agents and they need to be monitored for risks.
Several prior studies indicate that drugs congaing thiol group decrease the activity of mutagenic compounds in urine [23,24], so our results are in line with these studies and we tried to confirming of anti-mutagenic effect of oral Vitamin E on study population. The purpose of our study was to evaluate the anti-mutagenic effect of Vitamin E on possible mutagenic risk associated with antinoeplastic drugs. So because of ease to sampling of urine, we assessed the anti-mutagenic effects of Vitamin E of nursing urine samples. Some studies have suggested that, antioxidants especially Vitamin E, a chain-breaking lipophilic antioxidant found within biological membranes, is also physiological membrane-bund free radical scavenger [25], and our earlier observation indicates that Vitamin E as an effective chemopreventive agent is associated with diminution of oxidative stress [26]. Vitamin E ameliorates both the cardiac damage and carcinogenicity of the quinones adriamycin and daunomycin, which are mutagenic, carcinogenic, cause cardiac damage, and appear to be toxic because of free radical generation [27], and its Protective effects against radiation-induced DNA damage, mutation and dimethylhydrazine-induced carcinogenesis have also been observed [28]. In addition, it has been shown that Vitamin E in both the cases, by pretreatment/treatment has essential role on epithelial cells counts [29]. It was appeared that the urinary mutagenic activity will decrease by receiving Vitamin E. However, after Vitamin E consumption there were significantly depletion of urinary mutagenic activity in urine extracts among the exposed nursing personnel. Hence, since nurses are subjected to several anti-neoplastics drugs in job setting, which has been shown to have potent carcinogenic activities and the nursing resistance may decrease and they may encounter with oxidation stress of cellular components [30].
Conclusion
In summary the amount of mutagenic compounds in urine samples of oncology nurses depends on several factors likes protective equipments, the working period, the information and administration scores with regard to self-protection, and physiological states of a person, age and nutrition. Besides to these, a proper natural diet rich in antioxidants and anti-mutagenics such as Vitamin E should be considered. Also based on experimental evidence we propose that multiple higher therapeutic doses of Vitamin E supplements and increasing the length of treatment period will significantly diminish the level of mutagenic compounds in urine samples of oncology hospital nurses. Because of ease to sampling of urine, it can be inferred that urine sample may have essential diagnostic role among oncology nursing personnel.
t-test, p<0.05 significant for between groups
t-test, p<0.05 significant for between groups
t-test, p<0.05 significant for between groups