Empyema thoracis is defined as pus in the pleural space, is a serious complication of infection adjacent to/or within the chest that rarely resolves without appropriate medical therapy and drainage procedure. Empyema is usually a complication of pneumonia and in our country, tubercular empyema continues to be an important cause [1]. It is a common condition that is associated with considerable morbidity and mortality, whether treated or untreated [2]. The American Thoracic Society has described 3 stages of empyema, namely exudative, fibrinopurulent and organized, more or less based on the characteristics of the contents of the pleural cavity [3]. The exudative phase of empyema may be as short as 48 hours, it remains clinically challenging to plan therapeutic interventions based on duration of symptoms alone. This would suggest that, if therapeutic goal is to accomplish decortication in a minimally invasive fashion then, the initial treatment should be instituted early and with high expectations of success in the majority of cases, regardless of stage [4]. The optimal treatment of Empyema thoracis, especially in the fibrinopurulent phase, remains controversial so as to whether to go for a non-operative regimen (antibiotics, thoracocentesis and ICD tube drainage) or an early operative regimen (VATS and Decortication). While the former mode is less invasive and cheap, it is not clearly proved that it is better than the minimally invasive latter mode of treatment in terms of conversion into thoracotomy, morbidity and duration of hospital stay [5]. The advantages of minimal access VATS needs to be applied and proved beyond doubts as whether is it better than the treatment with ICD alone in the fibrinopurulent stage of empyema thoracis. This study was taken up to compare the efficacy of both treatment modalities in terms of morbidity, cost effectiveness, complications and treatment failure and to identify the optimal way of managing the condition.
Since the advent of minimally invasive surgical techniques in thoracic surgery in 1990, Video Assisted Thoracoscopic Surgery (VATS) has become the approach for many thoracic operations [6]. The major conceptual change leading to this development was the realization that adequate intrathoracic visualization can be obtained without a large incision because of improved video equipment and that manipulative techniques can often be modified to safely achieve the same results previously obtained only through large incisions [7]. In VATS the patient is placed in a lateral decubitus position under general anaethesia with one lung ventilation and the lung on the operative side being collapsed. Instruments employed for Laparoscopic work may be used to some extent, but is not convenient. For most purposes, the 0-30 degrees scopes are most useful and the flexible thoracoscope allows vision around corners.
Methodology
The study is a prospective study which was conducted over a period of 2 years (Dec 2008 to Nov 2010), in a tertiarry care Medical College Hospital. The source of data for the study are those patients admitted and diagnosed as Empyema thoracis in the fibrinopururlent stage, meeting the inclusion and exclusion criteria as mentioned below, during the study period in our hospital. The patients were from surgery, medicine, pulmonology, paediatrics and paediatric surgery departments. The data was collected as per the proforma comprising of history, clinical examination, investigations including imaging modalities and pleural fluid analysis and post-operative follow-up of the patients.
Inclusion criteria: Patients both adults and children diagnosed as Empyema thoracis in fibrinopurulent stage based on clinical examination, imaging modalities and pleural fluid analysis
Exclusion criteria: Empyema due to carcinoma, terminally ill patients, patients with severe respiratory distress at admission, patients not fit for surgery, patients with co morbid diseases like cardiac disorders and immunosuppression.
The selection of cases was based on history, clinical examination, imaging results, thoracocentesis results, general condition at the time of admission, co-morbid diseases and the patient’s willingness to join the study. A written consent was obtained from all the patients to undergo the necessary investigations and the type of surgical intervention. The patients diagnosed as empyema thoracis with the help of pleural fluid analysis and CT scan of thorax; who were in the second fibrinopurulent stage were selected for the study.
Sampling: Purposive sampling technique.
Study design: The study is a comparative study between the two modes of treatment. Both study groups consisted of 20 cases each.
Group A (ICD): First group of 20 patients were subjected to ICD tube insertion under local anaesthesia, following all the necessary standard precautions. The treatment was considered successful if the patient was symptom-free, good air entry on clinical examination, the repeat imaging showed no residual collection and good lung expansion and the drain was less than 20ml/day. The treatment was considered failure, if the patient did not improve clinically, as well as, radiologically and if he/she needed VATS/Thoracotomy to clear the disease.
Group B (VATS): Second group of 20 patients were subjected to primary VATS under General Anaesthesia. Pus was drained under vision and pleural plaques removed. Two chest tubes were inserted for each patient one anteriorly and the other posteriorly.The criteria for success were similar to that of Group A and the treatment was considered failure if the patient needed a thoracotomy later. The following variables were compared between the groups: duration of hospital stay, duration of chest tube in situ, cost of treatment, complications, treatment failure, mortality.
The statistical methods used are: Descriptive statistics, Frequencies, Crosstabs, Independent sample t-test.
Results
The descriptive variables such as age and sex were comparable in both the groups. The mean age in ICD group was 24 and that in VATS group was 34. There was no significant difference in the age distribution in either group. The most common presenting complaints in patients were breathlessness and cough. The commonest organism isolated in the pleural fluid was Streptococcus pneumoniae, followed by Staphylococcus aureus.
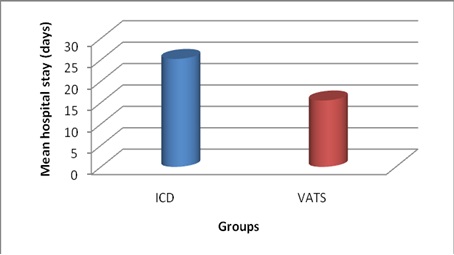
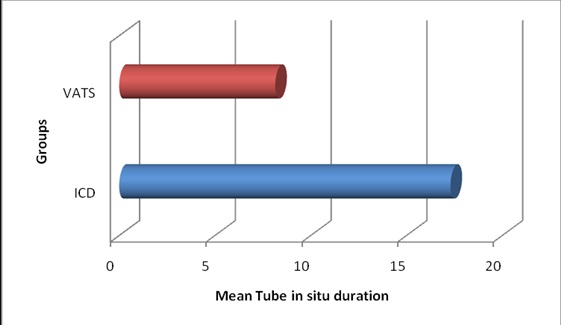
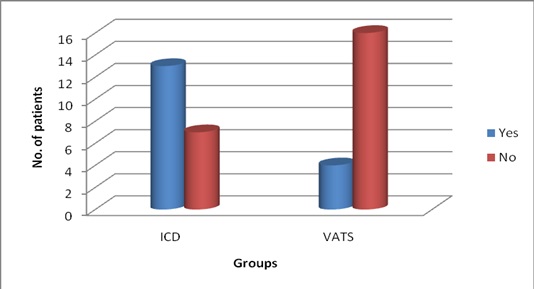
The mean duration of hospital stay in ICD group was 25.2, whereas, in VATS group it was only 15.5, which was statistically significant (p<0.05) as shown in [Table/Fig-2]. The mean duration of the chest tube in situ was 17.3 days in ICD group, whereas, it was only 8.1 days in that of VATS group, which was statistically significant (p<0.05) as shown in [Table/Fig-3]. The mean cost of the treatment was Rs.17,945 in ICD group, whereas, it was only Rs. 10,579 in that of VATS group, which was again statistically significant (p<0.05). The failure rate in ICD group was 65% (13/20) which were converted to VATS or open thoracotomy to completely cure the disease, thereby mean cost of the ICD group was significantly higher than primary VATS group. The major complications in ICD group were subcutaneous emphysema and the failure to cure the disease thus needing thoracotomy. The complications in the ICD group occurred in 80% of patients, the major being the failure. The major complications in VATS group were blood loss and air leak, which occurred in only 50% of patients. The difference in the complications rate was statistically significant (p<0.05). The failure rate in ICD group was 65% (13/20) compared to only 20% (4/20) in that VATS group, which was statistically significant (p<0.05), as shown in [Table/Fig-4]. There was no mortality in the study in either groups.
Bar diagram showing comparison of mean hospital stay (in days)
Bar diagram showing comparison of mean tube in situ duration (in days)
Bar diagram showing the comparison of failure (no. of patients)
Discussion
The optimal treatment of Empyema thoracis, especially in the fibrinopurulent phase remains controversial, so as to whether to go for a non-operative regimen (antibiotics, thoracocentesis and chest tube drainage) or an early operative regimen (Video assisted Thoracoscopic Surgery and Decortication). A detailed review of literature was done on this, which revealed VATS as a superior primary treatment in few studies, but not usually practised in routine. Despite the reported efficacy of different modalities in the treatment of empyema thoracis, an orderly approach to the empyema patient is unclear and cannot be easily established without prospective randomized trials enrolling equivalent patient groups [8]. This small study represents a prospective trial to randomize equivalent groups of empyema patients in the fibrinopurulent stage into two treatment groups comparing two commonly used therapies ICD and VATS as initial treatment. The observed results were in favour of VATS as the primary mode of treatment in the condition which was in accordance with previous studies as discussed below.
In our study, the mean duration of hospital stay in ICD group was 25.2, whereas, in VATS group it was only 15.5, which was statistically significant (p<0.05). This varied period was mainly an indicator of success or failure of the intervention done. The failure rate was more in ICD group and thus the duration of hospital stay was more in them leading to this result. This was in accordance with the results of a study done by Hurtgen M et al., [9]. The duration of chest tube in situ represents the efficacy of the intervention in clearing the pleural cavity of the collection and also represents the morbidity to the patient in terms of pain suffered and restricted mobility of the patient. The mean duration of the chest tube in situ was 17.3 days in ICD group, whereas, it was only 8.1 days in that of VATS group, which was statistically significant (p<0.05). One patient in ICD group had the longest duration of tube in situ i.e. 32 days - the tube was in situ for 20 days following the ICD and remaining 12 days subsequent to thoracotomy because of the failure of the primary intervention. In such cases of failures, this duration was calculated as the total number of days after which the patient is free of chest tubes. Our results were in accordance with the studies done by Wait et al., [10] and Doski et al., [11]. The cost of the treatment for the patients forms a major concern in patients, especially in a developing country like ours. The calculated cost did not include the cost of the investigations, such as CT because it was done for all patients and was in common the same amount.
The mean cost of the treatment was Rs. 17,945 in ICD group whereas it was only Rs. 10,579 in that of VATS group, which was again statistically significant (p<0.05). The patients who were cured with one primary intervention (success group) had to pay less. It has to be mentioned here, that patients of success group in ICD paid less than that of success patients in VATS group but considering the more number of failures in ICD group which were further treated with VATS or Thoracotomy, the mean cost in ICD group definitely goes up. The study done by Wait et al., also reported clinically relevant but not statistically significant differences in hospital costs ($16,642±2,841 vs $24,052±3,466, p=0.11), which also favoured the VATS group [10]. The main complications in ICD group were subcutaneous emphysema and the failure to cure the disease, thus needing thoracotomy. The complications in the ICD group occurred in 80% of patients, the major being the failure. The main complications in VATS group were blood loss and air leak which occurred in only 50% of the patients. The difference in the complications rate was statistically significant (p<0.05). It has to be noted here, that failure formed a major part of the complications rate in the ICD group (13/20), otherwise the complications such as subcutaneous emphysema were minor problems to manage. The failures in VATS group formed only a small part (4/20) of the complications rate, which needed open thoracotomy to clear the disease, other complications such as blood loss was not of a minor one and needed blood transfusions. The air leaks were also managed conservatively and none needed further intervention. In terms of failure rate, out of the 20 patients in the ICD group, only 7 had successful treatment after the primary intervention i.e. ICD, the remaining 13 had to undergo a second intervention in the form of VATS (5 patients) and Thoracotomy (8 patients). Whereas, in the VATS group 16 patients out of 20, had success with primary intervention and only 4 patients needed thoracotomy as the second intervention, thus the failure rate was 20% which is far less than that of the ICD group which was 65% (p<0.05). This is the most important issue in this study. The high success rate in VATS group attributes to drainage under vision and the advantage of clearing the pleural adhesions and giving the best chance of drainage of the collection in the pleural cavity which is not possible in the ICD group. Our results were supported by the studies done by done by Wait et al., [10] and Doski et al., [11]. Our study had no mortality in either groups.
Limitation
Our study is a prospective study with small sample size of patients. More studies including large randomised trials and prospective studies are needed to decide the proper management of the fibrinopurulent stage of empyema thoracis are needed for better understanding.
Conclusion
It may be concluded after the result findings observed, that it is very much evident that in the fibrinopurulent stage of Empyema thoracis, mere ICD tube insertion was associated with more morbidity and high chance of failure, requiring a longer stay and a second intervention to cure the disease. It was found that the duration of hospital stay, duration of chest tube in situ, cost of the treatment were less in the VATS group compared to the ICD tube group. VATS group also had lesser complications and lesser failure rate than the ICD tube group. Our study concludes that Video Assisted Thoracoscopic Surgery is better than conventional ICD tube insertion as a primary mode of treatment in the fibrinopurulent stage of Empyema thoracis.