The Thalassemia syndromes, considered the most common genetic disorder worldwide, are a heterogenous group of mandelian disorders, all characterized by a lack of/or decreased synthesis of either the alpha-globin chains (alpha thalassemia) or the beta-globin chains (beta thalassemia) of haemoglobin. Worldwide, frequency of thalassemia trait is about 3% [1], whereas in India the frequency ranges from 3%-18% & 1.3% in North and South India, respectively. Certain communities, such as Sindhis, Kutchis, Lohanas, Bhanusalis, Punjabis, Mahars, Agris, Gaud, Saraswats, Gowdas etc. in India have a higher frequency [2].
Apart from haemoglobin electrophoresis, where consistently elevated HbA2 levels are confirmatory, various other haematological investigations available are helpful in diagnosing beta thalassemia trait. However, these investigations are either expensive or time-consuming or cumbersome and often require sophisticated equipment. Hence, cannot be used as effective tools for population screening.
For screening purposes, a test which is inexpensive, requires a small amount of blood, does not require sophisticated equipment and can be applied on the population as a whole is preferred. These requirements are met by a modified osmotic fragility test “NESTROFT” (Naked Eye Single Tube Red Cell Osmotic Fragility Test), a test first described by Kattamis et al., [3]. The present study was undertaken, to evaluate the validity of NESTROFT as a screening test for the diagnosis of beta thalassemia trait in Northern India and to compare its findings with studies done in other parts of India and the World.
Material and Methods
The present study was conducted in the department of haematology, in a tertiary health care center, in the North Indian state of Punjab. The study comprised of patients of different age-groups, attending the outpatient departments of all the specialties presenting with various complaints associated with anemia. Haematological investigations such as haemogram and peripheral blood film (PBF) were done to confirm anemia and also to sub-type it. Subsequently, 111 cases who had a low haemoglobin (Hb) value, physiological for age and sex and were diagnosed as microcytic hypochromic anaemia on PBF were included in group I.
39 individuals comprising of family members (parents or siblings) of known cases of beta thalassemia major, irrespective of their haemoglobin and peripheral blood film findings were included in group II. Family members were selected because parents of thalassemia major patients carry beta thalassemia trait gene (100%) and the chances of carrying this gene in the siblings is also 50%.
NESTROFT and HbA2 levels were done in all the cases of group I and II.
Principle of NESTROFT
Normally, red cells put in saline solution begin to lyse at a saline concentration of 0.4-0.5% and lysis is complete at 0.32%. However, in beta thalssemia trait, due to alteration in osmotic resistance of the affected RBC’s due to volume/surface area ratio changes, [4] lysis begins at a saline concentration between 0.4-0.35% and it may not be completed even at 0.1% solution. NESTROFT is done at a saline concentration of 0.36%.
Material
0.36% buffered saline (BS) prepared by diluting 36ml of 1% buffered saline with 64ml of distilled water (DW) to make 100 ml (Test Reagent).
Procedure of the test
Two test tubes labelled as BS (2ml) and DW (2ml) were taken and a drop of blood was added to each of the tubes, which were then left undisturbed for half an hour at room temperature. Following this, contents of both tubes were gently shaken and held against a white paper on which a thin black line was drawn. The line was clearly visible through DW tube and if it was the same in BS tube; it was considered negative, otherwise test result was interpreted as positive [Table/Fig-1, 2].
Photograph showing negative NESTROFT
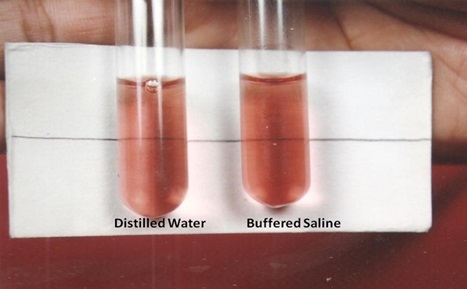
Photograph showing positive NESTROFT
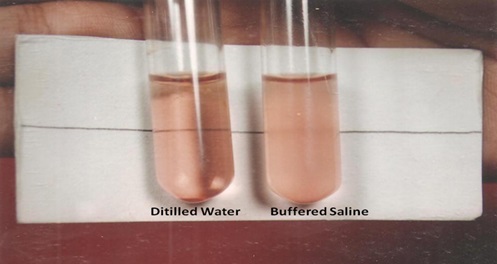
The tubes were left undisturbed for 3 hours. At the end of 3 hours, the DW tube was seen to be homogeneously pink with no sediments. In the BS tube the negative test showed similar findings as DW tube where as in a positive case, a clear supernatant and a sediment at bottom was observed [5].
HbA2 estimation was done by electrophoresis. The observations collected from the NESTROFT test and the Hb electrophoresis in both the sub-groups was recorded. By means of statistical analysis, an attempt was made to validate a correlation between NESTROFT positive samples and HbA2 levels in those cases.
Results
In the present study, the maximum number of patients belonged to 21 – 30 years of age group (53 patients; 35.53%) with range being 1.5 years to 80 years. Majority of the cases studied were females (86; 57.34%) [Table/Fig-3].
Distribution of age and sex
| Age Group (Year) | Total No. of Patients | Percentage (%) |
|---|
| Male | Female | Total | Male | Female | Total |
| 1-5 | 3 | 1 | 4 | 2 | 0.66 | 2.66 |
| 6-10 | 5 | 1 | 6 | 3.33 | 0.66 | 4 |
| 11-20 | 12 | 16 | 28 | 8 | 10.6 | 18.6 |
| 21-30 | 14 | 39 | 53 | 9.33 | 26 | 35.3 |
| 31-40 | 23 | 19 | 42 | 15.33 | 12.66 | 28 |
| 41-50 | 6 | 6 | 12 | 4 | 4 | 8 |
| >50 | 1 | 4 | 5 | 0.66 | 2.66 | 3.33 |
| Total | 64 | 86 | 150 | 42.66 | 57.34 | 100 |
Majority of the patients in the present study, had no obvious complaints related to anemia (52.67%), but weakness (38%) followed by fatigue and palpitations (2% each) were the next common complaints recorded.
Haematological investigations revealed that the majority of the patients in group I {77/111; (69.36%)} had Hb levels between 9.1-12g %. The mean value of Hb in this group was 9.9 ± 1.43g%. Though same applied to group II, {28/39 (71.80%)}, but the mean value of Hb for group II was 11.19 ± 1.31g% which was relatively higher than group I. 65/111 patients in group I had an RBC count between 4.1-5 million/cumm with the mean for the group being 4.77 ± 0.53 million/cumm. The mean RBC count for group II was much higher 6.11 ± 0.76 million/cumm. The mean PCV in group I (34.56 ± 4.48) was lower as compared to group II (38.69 ± 3.89). The mean value for MCV in group I was 72.2 ± 3.30 fl which was higher than that in group II 63.6 ± 5.49 fl. The mean value for MCH in group I was also higher (20.67 ± 1.34pg) as compared to group II (18.40 ± 2.15pg.) No significant difference was noted in the mean value of MCHC between the two groups [Table/Fig-4].
Mean values and standard deviation of various haematological parameters in Group I and Group II
| Parameter | Mean ± Sd |
|---|
| Group I | Group II |
| Hb (g/dl) | 9.90 ± 1.43 | 11.19 ± 1.31 |
| PCV | 34.56 ± 4.48 | 38.69 ± 3.89 |
| RBC count (million/cmm) | 4.77 ± 0.53 | 6.11 ± 0.76 |
| MCV (fl) | 72.2 ± 3.30 | 63.6 ± 5.49 |
| MCH (pg) | 20.67 ± 1.34 | 18.4 ± 2.15 |
| MCHC (g/dl) | 28.60 ± 1.08 | 28.92 ± 2.09 |
All the patients in the present study, except 4 in group II had microcytic hypochromic blood picture. These 4 patients had normocytic, normochromic blood picture.
NESTROFT was performed on all 150 selected cases. In group I, 20 (18%) cases out of 111 gave positive results with NESTROFT while 91 cases (82%) tested negative. In group II, 30 (76.92%) out of 39 cases tested positive with NESTROFT, while 9 (23.08%) gave a negative result.
HbA2 levels were also estimated in all 150 selected cases. Out of 20 NESTROFT positive cases in group I, only 3 had HbA2 levels more than 3.5%. The remaining 17 cases were false positive since HbA2 levels were less than 3.5%. In group II, all the 30 cases showing positive NESTROFT had HbA2 levels more than 3.5%. In this group, no false positive case was identified. None of the NESTROFT negative cases showed HbA2 levels more than 3.5%. So, all these cases were true negative [Table/Fig-5].
Calculation of Sensitivity, Specificity, Positive Predictive Value And Negative Predictive Value By 2x2 Contingency Table
| NESTROFT Positive | NESTROFT Negative |
|---|
| On Electrophoresis HbA2 levels >3.5% | 33 (True positive) | 0 (False negative) |
| On Electrophoresis HbA2 levels <3.5% | 17 (False positive) | 100 (True negative) |
Senstivity - 33/33±0x100= 100%
Specificity - 100/100±17x100= 85.47%
Positive predictive value- 33/33±17x100= 66%
Negative predictive value- 100/100±0x100= 100%
From the data thus obtained; sensitivity, specificity, positive and negative predictive values of NESTROFT were obtained. The test showed a sensitivity of 100%, specificity of 85.47%, a positive predictive value of 66% and a negative predictive value of 100%.[Table/Fig-5].
Discussion
Correct identification of beta thalassemia trait is especially important, as the management of a patient with beta thalassemia major is not only expensive (strain on the limited resources of developing countries), but also causes extreme misery to the patient and the family due to compromised quality of life. Also, as the red cell morphology in beta thalassemia trait is microcytic hypochromic; these patients are often misdiagnosed, as those suffering from iron deficiency anaemia and given unnecessary iron medication.
In this setting, a reliable screening test becomes a need of the hour for screening purposes. Many authors in India have found NESTROFT as a suitable test to identify carriers of beta thalassemia trait, especially in rural settings [6]. The present study was aimed at evaluating the usefulness of NESTROFT as a screening test for beta thalassemia trait in the North Indian state of Punjab and was carried out on 150 subjects.
In the present study, majority of the patients (52.67%) were clinically asymptomatic which corroborates with the work done by Mazza et al., [7] in African Americans, Knox Maculay et al., [8] in British population and Pootrakulate et al., [9] in the Chinese subjects where there was no appreciable symptomatology.
The changes seen in Hb values and other RBC indices and parameters of thalassemia trait cases in the present study, are compared individually with the work conducted by various researchers in India, as well as, internationally [Table/Fig-6 and 7]. A few salient points are discussed and highlighted briefly.
A comparision of various haematological parameters in beta thalassemia trait positive cases in present study with some earlier studies
| Study | Year | Hb (g/dl) | RBC COUNT (x1012/L) | PCV | MCV (fl) | MCH (pg) | MCHC (g/dl) |
|---|
| Pearson et al., [10] | 1973 | 11.16 ± 1.04 | - | - | 64.70 ±4.35 | 20.26 ±2.23 | 31.22 ± 0.34 |
| England & Fraser [11] | 1973 | 12 ± 1.3 | 6 ± 0.48 | 36.3 ± 4.2 | 60.8 ± 5.6 | 20.2 ± 1.9 | 33.2 ± 1.2 |
| ICMR Study [12] | 1993 | 12.2 ± 1.6 | 5.2 ± 0.7 | 38 ± 0.4 | 73 ± 10 | 23.7 ± 3.8 | 32.4 ± 1.9 |
| Madan et al., [13] | 1999 | 11.6 ± 1.6 | 5.56 ± 0.76 | - | 64.7 ± 4.8 | 20.6 ± 3.6 | - |
| Chakraborty et al., [14] | 2012 | 10.09±0.65 | 4.94±0.37 | 30.1±3.14 | 63.5±3.47 | 20.44±1.18 | 31.06±1.77 |
| Present study | 2013 | 11.23 ± 1.31 | 6.31 ± 0.67 | 38.97 ± 3.9 | 61.81± 2.92 | 17.82 ±1.39 | 28.83 ± 1.75 |
A comparative analysis of present study on NESTROFT with some previous studies is shown below
| Authors | Year | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|
| Mehta et al., [15] | 1988 | 95 | 82.1 | 73.1 | 97 |
| Raghwan et al., [16] | 1991 | 95.5 | 87 | 70.5 | 98.3 |
| Thomas et al., [17] | 1996 | 98.7 | 66.6 | 87 | 96.5 |
| Thool et al., [18] | 1998 | 95.2 | 100 | 100 | 83.3 |
| Maheshwari et al., [19] | 1999 | 91 | 95 | 55 | 99 |
| Suri & Sidhu [20] | 2001 | 97.7 | 71.7 | 51.9 | 99 |
| Bobhate et al., [21] | 2002 | 97.1 | 100 | 100 | 98 |
| Sirichotivakul et al., [22] | 2004 | 97.6 | 72.9 | 33.6 | 99.5 |
| Chakraborty et al., [14] | 2012 | 94.12 | 95.23 | 41.02 | 99.78 |
| Present study | 2013 | 100 | 85.47 | 66 | 100 |
The mean value of Hb in beta thalassemia trait positive cases in the present study, was slightly higher than Group I subjects (11.23 ± 1.31 g/dl). This is comparable to the work done by other researchers [Table/Fig-6].
In the study conducted, the mean value of the RBC count of Group I cases was lower than Group II (4.77±0.53million/cmm and 6.31±0.67million/cmm respectively). The mean value of Group I patients is comparable with the work done by most of the researchers [10–12,14]; Madan et al., however; found a count of 3.97±0.85 x 1012/ L, which is somewhat lower than that in the present study [13].
The mean value for PCV in both group I and II was quite comparable with the work done by various investigators [10–14].
In the present study, the mean values of MCV and MCH in group I (72.2 ± 3.3 fl, and 20.67 ± 1.34 pg) were compared with the other studies. It was found that while the mean value of MCV was slightly higher in the present study, the mean value of MCH was quite comparable [10–14]. The mean values of MCV and MCH in beta thalassemia trait cases (Group II) showed almost full concordance with the work conducted by other researchers [Table/Fig 6].
NESTROFT
In the present study, false positive cases recorded were 15.32%. Many researchers have reported the false positive rates to be ranging from 16.4% to 18.5% [14–22]. The false positive rates in the present study were quite comparable with work done elsewhere.
When Haematological parameters of these 17 false positive cases were computed [Table/Fig-8], it was found that the mean values of various parameters were nearer to those in Group I than those in Group II. So the haematological parameters pointed towards a diagnosis of iron deficiency anaemia rather than beta thalassemia trait [23].
Mean values and standard deviation of various haematological parameters in false NESTROFT positive cases
| Parameter | Mean ± Sd |
|---|
| Hb (g/dl) | 9.21 ± 1.38 |
| PCV | 32.68 ± 4.46 |
| RBC count (million/cmm) | 4.58 ± 0.45 |
| MCV (fl) | 71.13 ± 3.88 |
| MCH (pg) | 20.04 ± 1.42 |
| MCHC (g/dl) | 28.16 ± 1.02 |
Rest of the parameters of sensitivity, specificity, negative and positive predictive values computed in the present study, were also compared with the work done by various investigators [14–22]. It was found that very high rates of sensitivity (97.7-98.7%) were recorded in the works done by most of the researchers. This was also reflected in the present study (100%) [Table/Fig-7].
The rates of specificity reported by various investigators, range from 66.6% [16] to 100%. [20] The specificity rate of the present study (85.7%) is comparable with most of the other researchers [Table/Fig-7].
A wide variation in the rates of PPV has been quoted in the literature from 33.6% to 100%. Most of the researchers have quoted a PPV rate ranging from 51.9% to 73.1%. This is in concordance with the rate calculated in the present study (66.0%) [Table/Fig-7].
A high NPV is quoted by most of the researchers just like the work done in the current study [Table/Fig-7]. Only Thool et al., [18] has reported a slightly lower value of 83.3%.
Conclusion
From the above data, it is clear that NESTROFT is a highly sensitive and reasonable specific test for detection of beta thalassemia trait. The test is economical, as the cost of performing a single test is less than a rupee, quick, is easy to perform and does not require any sophisticated equipment. The solution once prepared has got a long shelf life. A high negative predictive value can reasonably rule out beta thalassemia trait cases. So, it should be adopted as a screening test for beta thalassemia trait, as it is not practical or feasible to employ HbA2 in every case of anemia in childhood.
A practical approach would be to perform NESTROFT on an accessible unmarried cohort of people, adolescents at school leaving or before starting college, or young adults starting a job. Positive cases could then be examined for raised HbA2 levels. When one considers the repeated yearly expenses of bringing up a child with thalassemia, preventing thalassemic births by diagnosing and counseling beta thalassemia trait carriers, becomes the more feasible and attractive alternative.
Senstivity - 33/33±0x100= 100%
Specificity - 100/100±17x100= 85.47%
Positive predictive value- 33/33±17x100= 66%
Negative predictive value- 100/100±0x100= 100%