Pain is the warning signal in almost all the inflammatory conditions. Arthritis is one of the inflammatory conditions involving damage to the joints leading to the pain and disability [1]. Knee pain due to Osteoarthritis (OA) is considered a highly prevalent disease among older persons [2]. Pain is one of the most disabling symptoms and can cause a significant aggravation of joint dysfunction. It reduces weight bearing and produce changes in the gait. Because of which it becomes difficult to carry out the day to day activities like walking, sitting, climbing, bending etc which impairs the quality of life in arthritic patient [3]. Therefore, symptomatic pain relief is essential. Current treatment strategies for arthritic inflammatory pain are based on use of steroids, non-steroidal anti-inflammatory and immunosuppressive drugs which provides the symptomatic relief [4]. Use of such drugs for longer duration may result into gastrointestinal upset, liver damage, kidney damage, opportunistic infections etc. They are very difficult to manage and many a times needs withdrawal [5]. Therefore, topical preparations are always considered to be superior in terms of adverse drug reactions and ease of application.
The seeds of Entada phaseoloides are available by different names in different countries like Gilla (Sanskrit), Hathibij (Hindi), Garambi (Marathi) and Gogo and are used worldwide for medicinal purpose. Paste of the seed pulp is used as an herbal medicine to reduce inflammation and pain of joints and lymph nodes. In our previous study anti-inflammatory and chondroprotective activity of these formulations are confirmed [6,7] and also reported to have emetic, anthelmintic and antimalignant activities [8,9]. So, the purpose of present study was to find out the analgesic potential of Entada phaseoloides seed pulp after topical application in animal models of knee joint arthritis.
Material and Methods
Institutional Animal Ethics Committee approval was taken before starting study. Entada phaseoloides seeds were obtained from M/s Gopal Govind Lokhande, 764, Budhwar Peth, Near Phadke Houdh, Pune 411002, India known vender of ayurvedic and unani medicine. Authenticated in Agharkar Research Institute, Pune-411004, India.
Drugs and Chemicals
Chemical required for the induction of pain Complete Freund’s adjuvant was obtained from Sigma Chemical Co., USA. Voveran Emulgel obtained from pharmacist of Novartis India Ltd-Mumbai, Polyethylene glycol 400 and 3350 -Analytical grade (ANALAR) from local supplier Sharad chemical Agencies, Pune., Pentobarbitone Sodium from Loba Chemie Industries, Mumbai.
Formulations Used
Ointment and paste are the different formulations used for the study.
(1) Paste of EP seeds powder [8,9]- Seed pulp (EP) was powdered and sieved through mill (No.80). Fresh paste was prepared by addition of water in the powder (5gm /5ml) and mixed to make uniform paste.
(2) Ointment of EP seeds powder [10,11]- Polyethylene Glycol (PEG) Ointment base was prepared by mixing PEG 400 (60%) & 3350 (40%). 5gm PEG mixture was warmed to 65oC, stirred while cooling until congealed. Five gm EP powder was incorporated in 5gm ointment base using tile and spatula. This preparation was divided into ten equal parts on the tile and one part is applied topically to each rat every day.
(3) Standard drug– Market preparation of Diclofenac ointment as Voveran Emulgel was used as positive control. One hundred gram of Voveran Emulgel contains 1.16g of diclofenac diethyl ammonium (equivalent to 1g DS), isopropyl alcohol, propylene glycol, perfume, Cream 45, and other additives.
Animals
Albino wistar rats of either sex weighing 150-200gms were used for the study. Housing 12 hours day and night cycle maintained. The rats were fed with commercial rat diet and Aquaguard water ad libitum. The experiment was designed and conducted in accordance with the ethical norms approved by Ministry of Social Justices and Empowerment, Government of India and Institutional Animal Ethical Committee Guidelines.
Groups
Rats were randomly divided into four groups of 8 animals in each. Group-I Control group, rats were treated with ointment base. Group-II EP paste, Group-III EP Ointment and Group-IV Diclofenac Sodium ointment rats received drug treatment according to groups. All topical formulations were gently applied to the left knee joint.
The paw pressure test was carried out by using the Randall and Selitto paw withdrawal method. Pressure was measured in grams force using an Ugo Basile Analgesy Meter by applying an increasing force to the left hind paw of rats until they reacted either by paw withdrawal or squealing. A threshold for nociception was determined for each animal at the start of the experiment to obtain baseline reading. Under light anesthesia intra-articular injection of 0.1ml of Complete Freund’s Adjuvant was given in left knee joint. All formulations were applied on the left Knee joint with at least 50 times gently rubbing in a circular manner. All the animals treated with drugs as per the groups and control animals were received ointment base application on the same day and for 72 hours. After 72 hours, nociceptive threshold (in gm) was estimated. Each animal served as its own control.
Parameters observed
Injection of Complete Freund’s adjuvant results into acute inflammatory reaction showing redness, swelling, pain and loss of function. So three important parameters observed. Localised inflammatory reaction & Gait was checked visually. Pain threshold was measured before and after drug treatment on analgesymeter.
Statistical Analysis
The statistical package, Graph Pad Prism 5 was used to analyse all results. Reduce gap between post hoc analysis and (Dunnett’s test) was used for analysis of data and for comparisons between treated and control groups, p<0.05 was considered significant.
Observation and Results
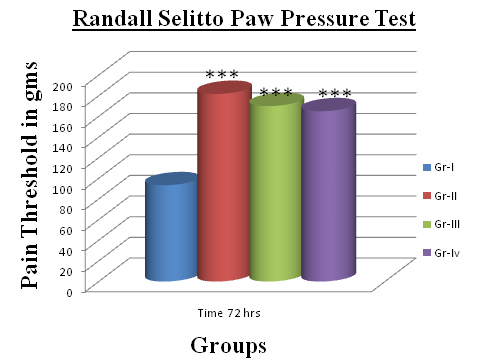
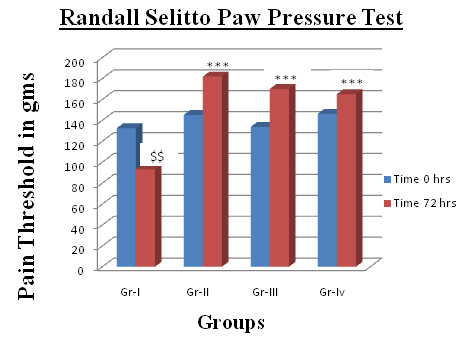
Difference between left and right knee joint was visible. Signs of inflammatory reaction due to Complete Freund’s adjuvant (CFA) were clearly observed. Visible local inflammatory changes such as swelling, redness on left knee within 24 hours were evident in control group. Visible local inflammatory changes such as swelling, redness were evident in control group. In Drug treated animals gait was minimally affected and locomotor activity was near to normal. In control group all the animals were limping and inactive. Pain threshold in control group was significantly $$ p<0.01 decreased at 72 hours [Table/Fig-1]. In comparison to control, there was significant (p<0.001) increase in the pain threshold in the all drug treated groups. EP ointment treated group showed comparable increase in pain threshold to that with Diclofenac ointment [Table/Fig-2]. Though not statistically significant, pain threshold of the paste treated group was higher than the Diclofenac ointment treated group.
Comparison of Pain Threshold at 72 Hours
*** p<0.001 Pain threshold of drug treated group in comparison with control
Comparison of Pain Threshold at 0 and 72 Hours
$$ p<0.01 Comparison of Pain threshold at 0 hours & 72 hours
*** p<0.001 Pain threshold of drug treated group in comparison with control
Discussion
The International Association for the Study of Pain defines pain as “an unpleasant sensory or emotional experience associated with actual or potential tissue damage” [14]. Reduction in pain threshold, restriction of movement, impairment of weight bearing and disturbed gait are the unavoidable consequence of knee joint arthritis [15].
Anti-nociceptive tests are designed in animals as models of the treatment of pathological pain in man, but they usually differ from the original in that the drug is given before the noxious stimulus [12]. Though indirect measures, weight bearing and gait analysis have the advantage that they are also used in the clinical setting to assess pain in patients with arthritis. Complete Freund’s Adjuvant (CFA)-induced arthritic rat model has extensively served as a laboratory model in the study of arthritic pain. Injection of CFA causes release of various mediators like PGs, LTs, cytokines etc. and hyperalgesia by sensitising pain receptors [16]. This leads to reduced pain threshold and restricted locomotion. Randall & Selitto who made use of the knowledge that inflammation increases sensitivity to pain and that this increased sensitivity is susceptible to modification by analgesics. According to the modifications done by the Perkins et al., model for testing the referred pain of knee joint arthritis was established [12].
In our previous study we have confirmed the anti-inflammatory and chondroprotective effect of topical preparations of Entada Phaseoloides seeds in rheumatoid and osteoarthritis model. EP Paste was found to be more useful in both these studies. Inflammation is always associated with pain, since EP formulations are effective in reducing inflammation they are also having analgesic action. In present study efforts are made to find out the efficacy of EP formulations for referred pain of knee joint arthritis with a suitable model like Randall Selitto paw pressure test.
EP is used topically as freshly prepared paste before use by practitioners of traditional & ayurvedic medicine for rheumatoid arthritis and osteoarthritis [8, 9]. The delivery of drug through the skin has long been a promising concept because of the ease of access, large surface area, vast exposure to the circulatory and lymphatic networks, and non invasive nature of the treatment [17,18]. The membrane-permeabilising activity showed by a large number of Saponins, whether triterpenoid or steroid. Strong foaming of aqueous solutions is the characteristic of Saponins. Foaming occurred in paste but not in ointment. This could be the factor responsible for increasing availability of EP in paste formulation leading to better efficacy [19,20]. EP ointment showed the anti-Nociceptive activity comparable to diclofenac sodium. Increase in the pain threshold indicative of improved weight bearing and locomotion.
Complete analysis of EP was done for active ingredient and it was observed that it consist of two sulphur containing amides ‘Entadamide A’ and ‘Entadamide B’ which are shown to inhibit 5-lipooxygenase (5 LOX) enzyme. [21,22] High concentrations of Leukotrienes have been observed in the arthritic joints. Inhibition of prostaglandins, leukotrienes and other mediators of inflammation, may be a probable cause of symptomatic relief in arthritic pain? Further studies are required to identify exact molecular mechanism of action of EP.
Conclusion
EP paste and ointment have potent analgesic actions and is comparable to that of diclofenac ointment. The present study revealed, both the formulations of EP are effective in reducing the knee joint arthritis induced referred pain. EP paste is more effective than ointment formulation. Study confirms the folklore use of EP for joint pain relief.