Most of the studies have utilized disk diffusion and agar dilution techniques for antimicrobial susceptibility testing, The disk diffusion method is cheap and easy to perform, but it offers only qualitative results. The dilution technique is time consuming and labour intensive [15]. Hence, an MIC determination is necessary, to confirm and to assess the degree of resistance [16].
This study was conducted to assess the prevalence of resistance to different groups of antibiotics in invasive S. pneumoniae and to verify the presence of MDR. Serogroups/types (SGTs) were identified and correlated with drug resistance.
Material and Methods
Study Site and Study Period
This prospective, active study was carried out in a tertiary care teaching hospital and research centre in Bangalore, from Feb 2009 to Feb 2011. S. pneumoniae which were isolated from children who were 28 days to ≤5 years old, who had IPD, were included in the study.
Bacterial Isolates
A total of 40 S. pneumoniae isolates were analyzed. Invasive isolates of S. pneumoniae were recovered from clinical specimens of normally sterile body sites such as, blood (n=36), CSF (n=3) and pleural fluid (n=1). The demographic, clinical, radiological and other investigational details of children was noted and scrutinized.
Identification, Serotyping and Antimicrobial Susceptibility Tests
Specimens (blood, CSF and pleural fluid) were inoculated into BACTEC Peds Plus/F blood culture bottles and they were incubated at 35°C in BACTECTM 9050 instrument (Becton Dickinson, Baltimore, USA) within 2 hours of their collections. The bottles were continuously agitated in the automated culture system for maximum recovery of organisms. Positive cultures were flagged by an indicator light and an audible alarm. The positive cultures were subcultured immediately onto sheep blood agar plates and the plates were incubated in a 5% CO2 incubator.
S. pneumoniae isolates were identified on the basis of colony morphology, gram staining, susceptibility to optochin and bile solubility tests, which were done by standard methods [17].
The strains were serotyped by the Quellung reaction according to the manufacturer’s recommendations. Test kits were obtained from Staten’s Serum Institute, Copenhagen, Denmark.
Antibiogram was performed by microdilution procedure using automated Siemens Microscan WalkAway® system with synergy panels (Siemens healthcare diagnostics Ltd, Frimley Camberley, UK). Standardized inoculum was prepared as per the manufacturer’s protocol. The antimicrobials which were tested were Penicillin, Erythromycin,Levofloxacin,Trimethoprim/Sulfamethoxazole(TMP-SMX), Vancomycin and Ceftriaxone. Strains were defined as Multidrug resistant if they showed resistance to three or more different groups of antibiotics.
MIC values of ≤0.06μg/ml defined Pneumococci as susceptible to penicillin, those of 0.12-1μg/ml defined them as Intermediately or relatively resistant and those of ≥2μg/ml defined them as highly resistant to oral penicillin V. For penicillin, parenteral (meningitis) doses of ≤0.06μg/ml and ≥0.12μg/ml could predict susceptibility and resistance respectively. MICs of ≤2μg/ml and ≥8μg/ml were the breakpoints for penicillin parenteral (nonmeningitis) [18].
All S. pneumoniae isolates which were identified in the local laboratory were confirmed by the Quintiles Central Laboratory at Singapore for quality control and assurance checks.
Ethics
This study was conducted in accordance with applicable laws and regulations. It was approved by the institutional review board and independent ethics committee. The subject’s parent(s) or legal guardian(s) completed written informed consent process.
Statistical Analysis
The statistical software, SPSS, v11.0 was used for the analysis of the data and for generation of figures and tables.
Results
Demographic Details
S. pneumoniae strains were isolated from children who were 28 days to ≤5 years old, who had IPD, who were living in south Bangalore region. Male to female ratio was 2.07:1 (Male= 27, 67.5% and Females= 13, 32.5%). Clinically, 25 (62.5%) patients were suffering from pneumonia, 11(27.4%) were suffering from bacteraemia, 4 (10%) were suffering from meningitis.
Serotype Distribution and Antibiogram
Forty pneumococcal strains were distributed among 11 SGTs [Table/Fig-1]. Four serotypes, 6 (n=10, 25%), 14 (n=7, 17.5%), 18 (n=5, 12.5%) and 5 (n=5, 12.5%), in order of prevalence, accounted for 67.5% (27/40) of all isolates. Serotypes 6 and 14 were the dominant types. Serotype 6 was most common in the age group of 28 days-20 months and type 18 was common in the age group of 21-40 months. There were differences in the distributions of serotypes among the different age groups. Serotypes 3, 4, 9 and 10 were seen only in 28 days-20 months age group and serotype 15 was seen only in age group of 41-60 months.
Age, Specimen and serotype distribution of 40 S. pneumoniae isolates
| Serotypes | 28days-20 months | 21-40 months | 41-60 months | Total |
|---|
| Blood | CSF* | PF† | Blood | CSF | PF | Blood | CSF | PF |
| 1 | 1 | - | - | 1 | - | - | 1 | - | - | 3 (7.5%) |
| 3 | - | 1 | - | - | - | - | - | - | - | 1 (2.5%) |
| 4 | 1 | - | - | - | - | - | - | - | - | 1 (2.5%) |
| 5 | 3 | - | - | 1 | - | - | 1 | - | - | 5 (12.5%) |
| 6 | 7 | 1 | - | 1 | - | - | - | - | 1 | 10 (25%) |
| 9 | 2 | - | - | - | - | - | - | - | - | 2 (5%) |
| 10 | 1 | - | - | - | - | - | - | - | - | 1 (2.5%) |
| 14 | 5 | 1 | - | 1 | - | - | - | - | - | 7 (17.5%) |
| 15 | - | - | - | - | - | - | 1 | - | - | 1 (2.5%) |
| 18 | 2 | - | - | 3 | - | - | - | - | - | 5 (12.5%) |
| 19 | 3 | - | - | 1 | - | - | - | - | - | 4(10%) |
| Total | 25 (62.5%) | 3 (7.5%) | - | 8 (20%) | - | - | 3 (7.5%) | - | 1 (2.5%) | 40 (100%) |
*Cerebrospinal fluid; †Pleural fluid
Penicillin resistance was seen in 14(35%) isolates. An intermediate penicillin resistance was seen in 9(22.5%) isolates who belonged predominantly to serotype 6 (n=6). A high level of penicillin resistance was seen in 5(12.5%) isolates who belonged largely to serotype 14 (n=3) [Table/Fig-2]. One isolate of serotype 6, which was obtained from pleural fluid, was highly resistant to penicillin. Resistance was not seen in any of the isolates which were recovered from CSF (n=3, serotypes 3, 6 and 14). The MIC range for penicillin was 0.016-4μg/ml.
Penicillin resistance and serotype distribution in IPD isolates
| Serotypes | IPD Isolates with Penicillin resistance |
|---|
| No. of Isolates | S* | I† | R‡ |
|---|
| 1 | 3 | 3 | - | - |
| 3 | 1 | - | 1 | - |
| 4 | 1 | 1 | - | - |
| 5 | 5 | 4 | 1 | - |
| 6 | 10 | 3 | 6 | 1 |
| 9 | 2 | - | 1 | 1 |
| 10 | 1 | 1 | - | - |
| 14 | 7 | 4 | - | 3 |
| 15 | 1 | 1 | - | - |
| 18 | 5 | 5 | - | - |
| 19 | 4 | 4 | - | - |
| Total | 40 (100%) | 26 (65%) | 9 (22.5%) | 5 (12.5%) |
*Susceptible; †Intermediate resistance; ‡High Resistance
Resistance was most common to TMP-SMX [Table/Fig-3]. 77.5% (n=31/40) of isolates were resistant to TMP-SMX, which predominantly belonged to SGTs 14 (n=5) and 6 (n=6). Among these isolates, high level and intermediate level resistances to TMP-SMX were observed in 42.5% (n=17/40) and 35% (n=14/40) isolates respectively.
Drug resistance pattern of S. pneumoniae
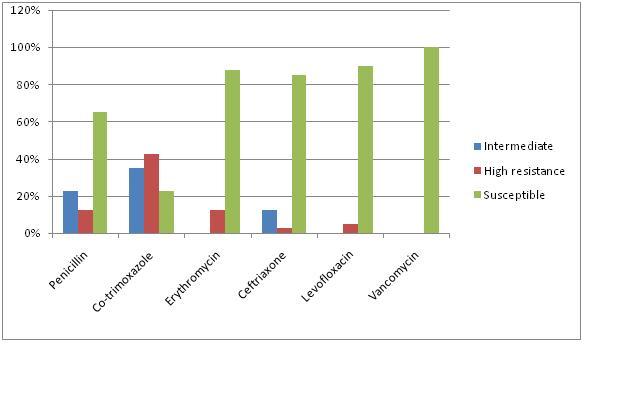
Ceftriaxone and Erythromycin resistant strains accounted for 15% and 12.5% strains, who belonged predominantly to SGTs 14 and 6, in that order. All the Erythromycin strains were highly resistant. Resistance to different antimicrobials, with serotypic distribution, has been represented in [Table/Fig-4].
IPD S. pneumoniae isolates- Serogroup/types and their antibiogram
| SGT | No. of Isolates | Penicillin | TMP-SMX* | Ceftriaxone | Erythromycin | Levofloxacin | Vancomycin |
|---|
| S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R |
|---|
| 1 | 3 | 3 | - | - | - | 3 | - | 3 | - | - | 3 | - | - | 3 | - | - | 3 | - | - |
| 3 | 1 | - | 1 | - | - | - | 1 | 1 | - | - | 1 | - | - | 1 | - | - | 1 | - | - |
| 4 | 1 | 1 | - | - | - | - | 1 | 1 | - | - | 1 | - | - | - | - | 1 | 1 | - | - |
| 5 | 5 | 4 | 1 | - | - | - | 5 | 5 | - | - | 5 | - | - | 5 | - | - | 5 | - | - |
| 6 | 10 | 3 | 6 | 1 | 4 | 5 | 1 | 8 | 1 | 1 | 7 | - | 3 | 10 | - | - | 10 | - | - |
| 9 | 2 | - | 1 | 1 | - | - | 2 | 2 | - | - | 1 | - | 1 | 1 | - | 1 | 2 | - | |
| 10 | 1 | 1 | - | - | - | 1 | - | 1 | - | - | 1 | - | - | 1 | - | - | 1 | - | - |
| 14 | 7 | 4 | - | 3 | 2 | 2 | 3 | 4 | 3 | - | 6 | - | 1 | 7 | - | - | 7 | - | - |
| 15 | 1 | 1 | - | - | - | 1 | - | 1 | - | - | 1 | - | - | 1 | - | - | 1 | - | - |
| 18 | 5 | 5 | - | - | 2 | 1 | 2 | 4 | 1 | - | 5 | - | - | 5 | - | - | 5 | - | - |
| 19 | 4 | 4 | - | - | 1 | 1 | 2 | 4 | - | - | 4 | - | - | 4 | - | - | 4 | - | - |
| Total | 40 | 26 | 9 | 5 | 9 | 14 | 17 | 34 | 5 | 1 | 35 | - | 5 | 38 | - | 2 | 40 | - | |
*Trimethoprim/Sulfamethoxazole.
Minimal resistance (5%) was observed to Levofloxacin. As was expected, Vancomycin was the antibiotic to which all the isolates were susceptible.
Multidrug Resistance
Multiple resistance was observed in 20% (n=8) of the strains. Penicillin, TMP-SMX and Ceftriaxone was the most common combination, which accounted for 62.5% (n=5/8). Intermediate and high penicillin resistances were observed in 3 and 5 isolates correspondingly. MDR strains belonged to SGTs 6 (n=3), 14 (n=3) and 9 (n=2), in descending order.
Discussion
Reports on increasing resistance to the commonly used antibiotics and the possible prevention of life threatening infections by using vaccines, monitoring of serotype prevalence and susceptibility pattern of S. pneumoniae have assumed great significance [19]. The serotypic distribution varies with geographical area, disease, age, socioeconomic conditions, season, vaccine usage and type of cohort [6]. Data on serotypic prevalence and antibiotic susceptibilities of invasive S. pneumoniae isolates in children who are ≤5years of age are limited in India.
SGTs involved in invasive infections in our study were dominated by type 6, followed by SGTs 14 and 18. The study of Kanungo and Rajalakshmi [19] which was conducted at Pondicherry in 2001 and a multicentre hospital surveillance study [8] which was done in 1999, have reported SGTs 6,1,23 and 6,1,19 respectively as the most prevalent isolates in the age group of ≤5years. Serotype 6 which is the most common serotype in developed countries [20], was also the most common one which was seen in this study, which accounted for 25% of all invasive isolates, which was in concurrence with other reports [19,8]. Reports from developing countries like Brazil [21] (SGTs 14,1 and 5), Bangladesh [22] (SGTs 7,12 and 14) and Nepal [23] (SGTs 1, 5 and 4) have shown a different pattern of invasive serotypic distribution. In these studies, serotype 6 was not the predominant isolate in young children (≤5 years). This brought into focus the different serotype prevalence patterns which were based on geography.
There are differences in the prevalence and the rank order of serotypes which are involved with invasive disease, depending on age groups. In young children who were 28 days-20 months old, S. pneumoniae type 6 was the most common one, whereas type 18 was predominant in the 21-40 months age group. However, we did not notice any significant difference in the distribution of serotype 1 across the age groups. Other Indian studies [19,8] have identified serotype 1 as the second most prevalent isolate (19% and 14%), which differed from our findings (7.5%). Serotype 1 which has largely disappeared from many developed countries, is also disappearing in this region of the country.
With the introduction of serotype specific, polyvalent, conjugate vaccines in India, determination of the major serotypes which cause invasive pneumococcal infections in different regions of India is of public health importance. 10 valent and 13valent Pneumococcal conjugate vaccines which are presently available in Indian market, contain 73% and 82% SGTs of our study respectively. Our study indicated that two SGTs, 10 and 15 were not covered by both vaccines.
The increase in the incidence and the spread of antibiotic resistant pneumococci has placed great emphasis on prompt and accurate recognition of pneumococcal resistant patterns. Our data clearly suggest an increase in the absolute number of the strains with reduced susceptibilities to penicillin in south Bangalore. Among 40 invasive pneumococcal strains, 14 (35%) had reduced susceptibility to penicillin (12.5% with high resistance and 22.5% with intermediate resistance). Analysis done, of studies done in India revealed that decreased susceptibilities to penicillin ranged from 1.3% to 18.3%. [8,28,19,24,11] Goyal et al., [11] (2.3%) and Chawla et al., [25] (4%) have reported low prevalences of high resistance to penicillin in S. pneumoniae in Delhi and Karnataka respectively. Increase in the percentage of strains with high resistance for penicillin (12.5%) in the present study was a matter of concern, as it could result in the spread of resistant strains to other locations. These strains could lead to higher rates of treatment failures and an increased economic burden. Therapeutic problems which are linked to this prevalence are compounded by the frequency of cross resistance to many other antibiotics.
Penicillin resistance was associated with a lower age being higher in age group of 28 days-20 months (78.5%), followed by that of 21-40 months (21.5%). These findings were concurrent with the observations of the ANSORP study [4]. Harbouring of S. pneumoniae in greater numbers in nasopharynx, frequent exposure to antimicrobial agents are the important risk factors for high percentage of penicillin resistance in children.
Studies [26,27] done in different parts of the world have shown that the high level penicillin resistant strains belonged primarily to SGTs 6, 19, 14 and 23 and that those with intermediate resistance belonged to SGTs 19 and 14. Lalitha et al., [28] have reported that the intermediate penicillin resistant strains of S. pneumoniae from the surveillance programme belonged predominantly to SGTs 14 and 19 and that SGT 1 was most prevalent in Kanungo’s and Rajalakshmi’s study [19]. Information related to SGT pattern of high level penicillin resistance from India is scant [8,19]. In the study done by us, high level penicillin resistant strains (n=5, 12.5%) mainly belonged to SGT 14 (n=3, 60%) and intermediate resistant strains (n=9, 22.5%) belonged largely to SGT 6 (n=6, 66.6%). This underlines the need for a constant surveillance and reporting of the findings [8,19].
In this study, 10-valent PCV and 13-valent PCV covered 80% and 100% SGTs of the penicillin resistant strains respectively, thus indicating the potential usefulness of the vaccination in Bangalore population. Since Pneumococci have the genetic capacity to switch serotypes by horizontal transfer, recombination or other genetic events, knowledge on the frequencies of these serotypic exchanges is important, to predict the long term efficacy of vaccines.
TMP-SMX has been widely used for upper respiratory tract infections, because of its broad coverage, synergetic effects and low cost. TMP-SMX functions by inhibiting dihydrofolate reductase and dihydropteroate synthase. Alteration of these genes by mutations or acquisition of exogenous genes, leads to resistance. The rate of pneumococcal resistance to TMP-SMX has increased significantly in India [24,19,25,11], from 21.8% to 61.7% between 1996-2002. A high rate of resistance (77.5%) to TMP-SMX was observed in our study as compared to that which was seen in other studies done in India. Alarming levels of TMP-SMX resistances raise the question as to whether WHO recommendations on use of TMP-SMX as first line of treatment of choice in upper respiratory tract infections needs to be revised, based on local data.
85.7% (n=12) of penicillin resistant strains (intermediate resistance n=7, high resistance n=5) had co-resistance to TMP-SMX. All the 6 strains which were resistant to Ceftriaxone and all the 5 which were resistant to Erythromycin were also resistant to TMP-SMX. The emergence of such strains is of particular concern in the treatment of pneumococcal meningitis, because ceftriaxone is an important component of the combination regimens which are used for pneumococcal meningitis [4].
Multidrug resistance was observed in 8 strains, all of which had reduced susceptibilities to penicillin. Penicillin resistance is an important marker for multidrug resistant phenotypes. Each of high penicillin resistant strains in our study exhibited multidrug resistance. To the best of our understanding, this is the first report from India, which has described this high resistance profiles of S. pneumoniae strains. The increased rate of resistance to these antibiotics can be possibly correlated with the wide use of these antibiotics in communities, because of their dose convenience, cost effectiveness, and also, their easy availability over the counter [23]. This is a matter of great concern, as it results in higher morbidity, mortality.
Our data clearly documents high rate of MDR, changing trend of antibiogram of S. pneumoniae, with a distinctive increase in the prevalence rate and resistance level of penicillin and other drugs. The strength of this study lies in its ability in determining different dynamics of serotype-specific IPD and drug resistance in specific age groups. The sensitivity resistant patterns which are identified, will help the clinicians in appropriately planning treatment regimen. Our study indicated that a majority (82%) of IPD cases could be prevented with the use of 13valent PCV.
Due to the diversity in populations in different parts of India and also the living conditions, our findings cannot be generalized. Owing to small number of isolates in this study, there are limitations to our findings, that may reduce the generalizability of our results.
Conclusion
The study identified a high prevalence of penicillin resistance and MDR in invasive S. pneumoniae among those children who were aged below five years. A continuous surveillance of serotypes and antimicrobial resistance patterns of pneumococci in multicentric studies which involve rural and urban areas, is needed. Appropriate antibiotic treatment plans and use of pneumococcal vaccinationa are essential, to decrease morbidity and mortality.