The irritation or inflammation of the meninges, the covering of brain and spinal cord, is known as meningitis. Mostly, the leptomeninges (pia and archnoid) are affected rather than the dura matter. The latter is involved in a localized or patchy manner and it is usually a result of a direct spread of infection from the adjacent bone.
Acute bacterial meningitis (ABM) is a medical emergency, which warrants an early diagnosis and an aggressive management. Most often, therapy for bacterial meningitis has to be initiated before the aetiology is known. Acute bacterial meningitis remains a major cause of mortality and long-term neurological sequelae worldwide. Despite the availability of potent newer antibiotics, the mortality rate caused by acute bacterial meningitis remains significantly high in India and other developing countries, which ranges from 16 - 32% [1,2].
Studies which have been done in various geographical regions have revealed that meningitis is caused by many different pathogens, depending on the patients’ age groups. Among neonates, Group B and non-Group B Streptococcus species (49%), Escherichia coli (18%) and Listeria monocytogenes (7%) are the most common pathogens which are seen. The meningitis-causing pathogens which are found in infant and child age groups are Haemophilus influenzae (40-60%), Neisseria meningitidis (25-40%) and Streptococcus pneumoniae (10-20%). The common pathogens which are encountered in adult meningitis are Streptococcus pneumoniae (30-50%), Neisseria meningitidis (10-35%), Staphylococci (5-15%), other Streptococcus species, Haemophilus influenzae (1-3%), Gram negative bacilli (1-10%) and Listeria monocytogenes [2–6]. There is a need for a periodic review of bacterial meningitis worldwide, since the pathogens which are responsible for the infection may vary with time, geography and patients’ ages [7,8]. Increased awareness, the availability and the usage of vaccines may also be responsible for a change in the epidemiological patterns of these pathogens. A delay in diagnosis and initiation of antimicrobial therapy can result in a poor outcome of the disease [9]. Since clinical signs of meningitis are not always reliable, a laboratory support is imperative, to achieve an early diagnosis. The emergence of antimicrobial resistance has added to this problem, and current recommendations are to identify targets for immunization, formulate preventive strategies and to carry out a rational empirical treatment, especially for potentially fatal bacterial meningitis [1,7–9].
The aim of the present study was to analyze the bacterial profiles and antimicrobial susceptibility patterns of the isolates which were obtained from CSF of patients with acute bacterial meningitis in the given area.
Material and Methods
This study, which was approved by our Institutional Ethics Committee, was conducted in the Department of Microbiology, Patna Medical College, Patna, India, during the period from August 2011 to December 2012. Informed consents were obtained from the patients’ relatives for their inclusion in the study.
Two hundred and fifty two suspected cases of acute bacterial meningitis, who were admitted to various wards of our hospital, were included in this study. The patients were assessed for acute meningitis, based on the following clinical signs and symptoms: headache (not relieved by analgesics), nausea or projectile vomiting (not relieved by antiemetics), low or high-grade fever of acute onset which lasted for several days, delirium or altered consciousness, mental apathy, neck rigidity, a positive Kernig’s sign, ophisthotonos, etc. Cases of post-traumatic and post-cranial surgery meningitis were excluded from the study group. Only one representative CSF sample from every patient was included and consecutive CSF samples from the same patient were ignored for the purpose of the study. Lumbar puncture was performed aseptically on the patients and cerebrospinal fluid (CSF) was collected in sterile screw capped containers. Altogether, 252 samples of CSF were initially subjected to a naked-eye examination for gross appearance, and they were processed further for cell counts, biochemical analysis, gram staining, culture, antigen detection by latex agglutination test (LAT) and antibiotic susceptibility tests.
Routine total and differential cell counts were obtained for each sample by using a standard haemocytometer. Protein and sugar levels in the samples were estimated by using a standard, semi-automated biochemistry analyzer. CSF sediment smears were gram stained and they were examined for the presence of microbes, if any. Culture was performed on 5% sheep blood agar, Chocolate agar and Mac Conkey’s agar. The isolates were identified by standard bacteriologic, biochemical and serologic methods [10]. Detection of soluble antigens of H. influenzae (type b), S. pneumoniae and N. meningitidis in CSF was done by latex agglutination test, by using commercially available kit from Tulip Diagnostics Pvt. Ltd, India. Antigen detection was performed only in 52 cases with culture negative CSF samples.
Antimicrobial sensitivity test was performed on Mueller Hinton agar by the Kirby Bauer disk diffusion method [11]. The antibiotics which were used were ampicillin (10 μg), co-trimoxazole (25 μg), cefoxitin (30 μg), cephotaxime (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg), amikacin (10 μg), ciprofloxacin (30 μg), levofloxacin (5 μg), erythromycin (30 μg), penicillin G (10 units), amoxicillin + clavulanic acid (20/10 μg), piperacillin + tazobactam (100/10 μg), imipenem (10 μg) and vancomycin (30 μg), which were obtained from Hi Media Laboratories, Mumbai, India. The results were interpreted as per NCCLS-2000 recommendations [12].
Results
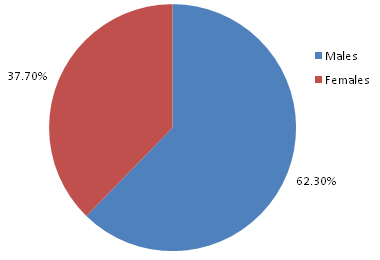
Of the 252 patients who were clinically suspected of having acute bacterial meningitis, 157 (62.3%) were males and 95 (37.7%) were females [Table/Fig-1]. The most common age group of presentation was 12-60 years (80.2%), while 13.4% patients were <12 years of age [Table/Fig-2]. The patients showed variable symptomatologies which ranged from headache (99%), fever (96.5%), nausea (90.3%), projectile vomiting (90%), neck rigidity (89.2%), to delirium/altered consciousness (16.3%), seizures (4%), opisthotonus (3.3%), hypotension (6.2%) and skin rash (12.6%). However, bleeding or other complications were not found in any patient.
Percentage distribution of patients of ABM based on sex (n=252)
Distribution of ABM patients based on age groups (n=252)
| Age group(years) | Number of patients | Percentage (%) |
|---|
| 0-2 | 20 | 7.9 |
| 2-5 | 6 | 2.3 |
| 5-12 | 8 | 3.2 |
| 12-60 | 202 | 80.2 |
| >60 | 16 | 6.4 |
| Total | 252 | 100 |
[Table/Fig-3] shows the correlation between smear and culture findings in patients with ABM. The bacterial pathogen could be demonstrated in gram stained smears of 162 (64.3%) CSF samples, while CSF culture yielded positive growth in 200 (79.4%) patients.
Correlation of smear and culture findings in ABM (n=252)
| Culture + | Culture - | Total |
|---|
| Smear + | 148 | 14 | 162 (64.3) |
| Smear - | 52 | 38 | 90 (35.7) |
| Total | 200 (79.4) | 52 (20.6) | 252 (100) |
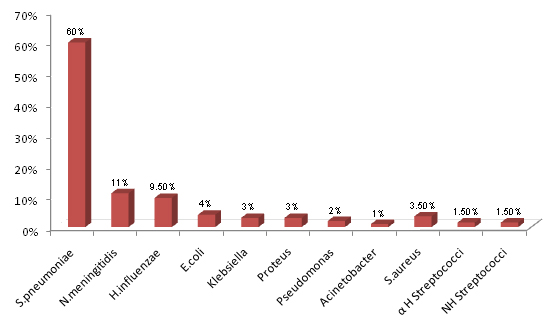
Streptococcus pneumoniae was the most common pathogen which was isolated in 120 (60%) culture positive cases, followed by Neisseria meningitidis which was isolated in 22 (11%) cases and Haemophilus influenzae which was isolated in 19 (9.5%) cases. The other pathogens which were isolated less commonly were Escherichia coli, Klebsiella spp., Proteus spp., Pseudomonas spp. and Acinetobacter spp. in the gram negative group, and Staphylococcus aureus and Other Streptococci spp. in the gram positive category [Table/Fig-4].
Etiological agents isolated in culture positive CSF samples (n=200)
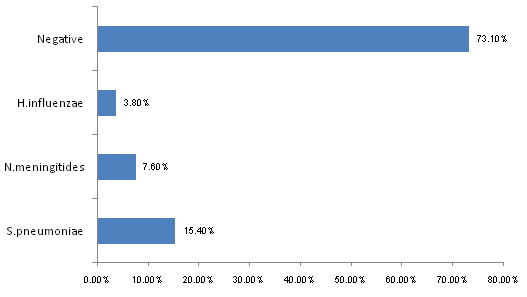
Among the 52 culture negative cases, an additional 8 (15.4%) cases of S.pneumoniae, 4 (7.6%) cases of N.meningitidis and 2 (3.8%) cases of H.influenzae could be detected by latex agglutination test for antigens of these pathogens. The remaining 38 out of 52 (73.1%) cases were those wherein the CSF samples were negative in gram stained smear examination, culture and LAT [Table/Fig-5]. These could have been possibly caused by pathogens which were not detectable by the methods which were employed in this study.
Culture negative samples of CSF positive for antigens of S.pneumoniae, N.meningitidis and H.influenzae by LAT (n=52)
The cell counts of the CSF samples ranged from no cells to sheets of cells, which could not be counted on the haemocytometer. A predominance of neutrophils was the common feature which was seen in all cases with high cell counts [Table/Fig-6]. The mean sugar level in the CSF samples were tested was 32.2 ± 3.4 mg/dl, while a high mean level of protein (90.2 ± 11.5 mg/dl) was detected in these samples.
Counts in relation to pathogens isolated in CSF (n=222)
| Organisms | < 200 cells/mm3 | 200-1000 cells/mm3 | > 1000 cells/mm3 | Not recorded |
|---|
| S.pneumoniae | 20 | 38 | 70 | 2 |
| N.meningitidis | 1 | 9 | 20 | 0 |
| H.influenzae | 2 | 8 | 12 | 1 |
| Gram negative bacilli | 1 | 4 | 20 | 1 |
| S.aureus | 1 | 1 | 4 | 1 |
| Other Streptococci | 0 | 1 | 5 | 0 |
Antibiotic sensitivity tests were performed on the isolates of S.pneumoniae, Other Streptococci, S.aureus, E.coli, Klebsiella spp., Proteus spp., Pseudomonas spp. and Acinetobacter spp. All the 120 isolates of S. pneumoniae were sensitive to penicillin G and vancomycin. The 7 isolates of Staphylococcus aureus which were obtained in this study showed low sensitivity towards ampicillin (21.78%) but relatively higher sensitivities to the other antibiotics. The other Streptococcal isolates showed variable sensitivities towards the antibiotics which were tested, as has been depicted in [Table/Fig-7]. All gram positive isolates were sensitive to vancomycin (100.0%).
Antimicrobial sensitivity pattern of S.aureus and Other Streptococci isolated in CSF
| Antimicrobial agents | S. aureus (n=7) | Other Streptococci (n=6) |
|---|
| Penicillin G | 100% | 100% |
| Amikacin | 81.73% | 80.0% |
| Amoxicillin+clavulanate | 100.0% | 100.0% |
| Ampicillin | 21.78% | 60.0% |
| Cefotaxime | 61.0% | 100.0% |
| Ceftazidime | 61.0% | 100.0% |
| Ceftriaxone | 61.0% | 100.0% |
| Cephoxitin | 56.52% | 40.0% |
| Ciprofloxacin | 87.0% | 100.0% |
| Co-trimoxazole | 34.83% | 60.0% |
| Erythromycin | 65.65% | 100.0% |
| Ciprofloxacin | 85.0% | 100.0% |
| Levofloxacin | 86.74% | 100.0% |
| Vancomycin | 100.0% | 100.0% |
The antimicrobial sensitivity patterns of the gram negative isolates have been summarized in [Table/Fig-8]. The E. coli isolates showed lowest sensitivity towards ampicillin (20.0%), cotrimoxazole and cephalosporins (22.5-34.16%), whereas their sensitivities to amoxycillin+clavulanic acid (87.5%) and piperacillin+tazobactum (90.0%) were relatively much higher.
Antimicrobial sensitivity pattern of gram negative CSF isolates
| Antimicrobial agent | E. coli (n=8) | Klebsiella spp. (n=6) | Proteus spp. (n=6) | Pseudomonas spp. (n=4) | Acinetobacter spp. (n=2) |
|---|
| Amikacin | 49.1 | 45.71 | 25.0 | 40.0 | 0 |
| Ampicillin | 20.0 | 28.57 | 0 | 0 | 0 |
| Amoxicillin +clavulanate | 87.5 | 85.71 | 75.0 | 60.0 | 100.0 |
| Co-trimoxazole | 22.5 | 22.5 | 0 | 0 | 0 |
| Cefotaxime | 34.16 | 37.14 | 25.0 | 0 | 0 |
| Ceftazidime | 34.16 | 37.14 | 25.0 | 0 | 0 |
| Ceftriaxone | 34.16 | 37.14 | 25.0 | 0 | 0 |
| Cephoxitin | 25.8 | 31.42 | 0 | 0 | 0 |
| Ciprofloxacin | 54.16 | 57.14 | 25.0 | 40.0 | 0 |
| Levofloxacin | 55.0 | 62.85 | 25.0 | 40.0 | 0 |
| Piperacillin+ tazobactum | 90.0 | 85.71 | 75.0 | 80.0 | 100.0 |
| Imepenem | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
The isolates of Klebsiella showed low sensitivities against ampicillin (28.57%), cotrimoxazole (22.5%) and cephalosporins (31.42-37.14%) and high sensitivities to amoxycillin+clavulanic acid (85.71%) and piperacillin+tazobactum (85.71%). The other isolates showed variable sensitivities to the various antibiotics which were tested. Overall, all the gram negative isolates were sensitive to imipenem (100.0%).
Of the 252 patients who were clinically diagnosed as suffering from ABM, 22 (8.7%) patients expired during the course of the study. N.meningitidis accounted for 9 (40.9%) cases of mortality, followed by S.pneumoniae which accounted for 3 (13.6%) cases and H.influenzea which accounted for 1 (4.5%) case. In the remaining 9 (40.9%) mortality cases, the organism could not be identified by the various microbiological tests which were used in the study.
Discussion
Meningitis can be broadly classified as pyogenic, granulomatous, and lymphocytic. Acute bacterial or pyogenic meningitis is a potentially life threatening disease that consists of inflammation of the meninges and the underlying subarachnoid CSF. Pyogenic meningitis can be accurately and rapidly diagnosed by gram staining, and some studies have reported sensitivities of 60-90% and specificities of >97% of CSF gram staining in the diagnosis of ABM [2,6,13,14].
In the present study, males were found to suffer from ABM 1.65 times more frequently than females. This male preponderance which is seen with this disease, has also been reported in several previous studies [6,8,15]. A majority of patients (86.6%) in our study group were adults, while only 13.4 % patients were from the paediatric age group, which was similar to the findings of other workers [1,16].
Though the common pathogens which are associated with acute bacterial meningitis are S. pneumoniae, H. influenzae and N. meningitidis, the aetiological agents and their relative frequencies may vary in different geographical areas. Changing trends in the epidemiology of ABM are being observed and reported worldwide. Bacterial meningitis is being reported predominantly in adults in USA, because of the immunization practices which have been adopted and also, because of a relative increase in frequency of nosocomial meningitis. In north America and Europe, S. pneumoniae and N. meningitidis remain important pathogens in children and young adults, mainly due to a vaccine related decline in H. influenzae disease. Group B Streptococcus is the most common pathogen which is associated with meningitis in newborns. Listeria monocytogenes is also being recognized as an important cause of meningitis in newborns and in the elderly in the United States [17]. As compared to that which is seen in western studies, the relative incidence of meningitis which is caused by H. influenzae, N. meningitidis and Listeria is less in south-east Asia. On the contrary,
Gram negative bacilli such as E.coli, Klebsiella pneumonia, Proteus and Pseudomonas aeruginosa are inc reasingly being reported in cases of meningitis, especially among the elderly and in patients with cirrhosis, diabetes and malignancies [1,8,17].
Streptococcus pneumonia, Neisseria meningitidis and Haemophilus influenzae were the three most common pathogens which were isolated in this study [Table/Fig-4 and 5]. These findings were in accordance with the isolation rates for these pathogens which were reported in several earlier studies [3,6,17,18], but they were different from the observations of Chinchankar et al., who found a very high incidence of H.influenzae in ABM cases in paediatric age group [4]. We found meningococcal meningitis in 9.5% cases of ABM, which was in contrast to its low incidence which was reported in other Indian studies [1,4]. The Gram negative bacilli, as aetiological agents in ABM, as has been reported in this study and in various other studies, [4,6,7] account for a minor percentage of the cases. More data from systematic studies all over India needs to be analyzed, to comment as to whether meningitis which is caused by Gram negative bacilli other than N.meningitidis is on the increase, which is similar to the trend which is found in some other countries. No case of Listeria monocytogenes meningitis was seen in this study. Though the incidence is low in most of the Indian studies, it should be considered especially in the elderly and immunocompromised patients, since it is known to be resistant to third generation cephalosporins which are used in the empirical treatment of bacterial meningitis.
A simple gram stained smear can offer immediate clues to aid a diagnosis of pyogenic meningitis, as has been reported by several workers [2,13,14]. In the present study, examination of gram stained CSF smears helped in the diagnosis of ABM in 164 (64.3%) cases. The yield of bacteria on gram staining depends on several factors like the number of organisms which are present, prior use of antibiotics, technique which is used for smear preparation (centrifuged deposit, cytospin, direct smear, etc.), staining techniques and the observers’ skills and experiences. Despite variable gram stained smear positivities which are found in CSF samples and the fact that a negative gram staining does not rule out infection, the importance of a positive smear cannot be ruled out, especially in developing countries, where financial constraints limit the use of other rapid diagnostic tests in diagnosis of this potentially fatal infection.
One hundred fourty eight (58.7%) smear positive cases in this study were found to be positive for culture as well. An additional 52 (20.6%) smear negative but culture positive cases were detected in this study.
All 52 (20.6%) culture negative cases were subjected to LAT, through which an additional 14 (5.5%) cases of ABM could be diagnosed. The remaining microbiologically negative samples could possibly have been caused by pathogens which are not detectable by these routine methods. Our findings of gram stained smear examinations, culture positivity and LAT of the CSF samples were in accordance with the observations of several other workers [1,19,20]. Several workers have questioned the clinical usefulness of antigen detection tests, which have explained that a negative test did not rule out infection and that false positive results could lead to an unnecessary prolonged course of antibiotics, lengthened hospital stays and in some cases, important clinical complications. It is possible that the antiserum in diagnostic LAT kits does not detect all the capsular serotypes which are prevalent in a particular geographical area or probably, as the yet unrecognized serotypes are the causative agents in such cases, as has been reported in several studies [20]. However, several studies [1,4,19] have advocated the usefulness of LAT, especially in pretreated cases and to differentiate partially treated pyogenic meningitis from tuberculous meningitis, which is rampant in India. Despite its drawbacks, we found LAT to be a simple, rapid procedure which was suitable to be used as an adjunct laboratory test, but it needs to be interpreted cautiously, taking the patient’s clinical condition and several other factors into consideration. In developing countries like India, where many laboratories lack facilities for culture and other elaborate investigations, LAT can help in establishing the diagnosis of ABM. However, the high costs of LAT kits remain a prohibitive factor in its routine use in most laboratories.
We found neutrophilic pleocytosis to be a consistent finding in a majority of cases of ABM. Raised levels of CSF proteins and decreased CSF sugar levels were observed by us, which were similar to the findings of other workers [1,8,9].
In this study, all the isolates of S. pneumoniae were sensitive to penicillin G and vancomycin. The other Streptococcal isolates showed variable sensitivities towards the antibiotics which were tested. Some studies have reported emergence of penicillin resistance in Streptococci [21]. All the gram negative isolates were sensitive to imipenem. The overall sensitivity patterns of the isolates, as was observed by us, were similar to the findings of previous Indian studies [1,8,22].
The final diagnosis of ABM depends upon a comprehensive analysis of CSF smears, culture, LAT, cytological, biochemical and clinical findings of the cases, and a single test or parameter cannot be used as a benchmark, to decide the course of management in the patient. However, empirical therapy is advocated, considering the potentially high rates of mortality in these patients. The use of antibiotics should be judicious and it should be based on current knowledge of the prevailing susceptibility patterns of the pathogens in an area.
Conclusion
The spectrum of organisms which cause acute bacterial meningitis varies with time, geography and patients’ ages. Since clinical signs of meningitis are not always reliable, a laboratory support is imperative, to achieve an early diagnosis. The emergence of antimicrobial resistance has added to this problem, and current recommendations are to identify targets for immunization, formulate preventive strategies and to carry out rational empirical treatment, especially for potentially fatal bacterial meningitis.
Simple, rapid, inexpensive tests like gram staining remain the significant means for diagnosing ABM in developing countries. To increase the cost-effectiveness in a resource limited setting, LAT for pneumococcal antigen should be performed first, since it is the most common pathogen which causes ABM in all age groups. Streptococcus pneumoniae remains the major aetiological agent of ABM, both in adults and children, not only in India, but worldwide. Meningitis which is caused by H. influenzae has almost been eliminated from the western world, following routine vaccination with Hib conjugate vaccine. Introduction of conjugate vaccines against Streptococcus pneumoniae can reduce the burden of childhood meningitis and it may produce a herd immunity among adults.